Question: A copper, Cu(s), electrode is immersed in a solution that is 1.00 M in ammonia, NH3, and 1.00 M in tetraamminecopper(II). [Cu(NH3)4]2+ If a
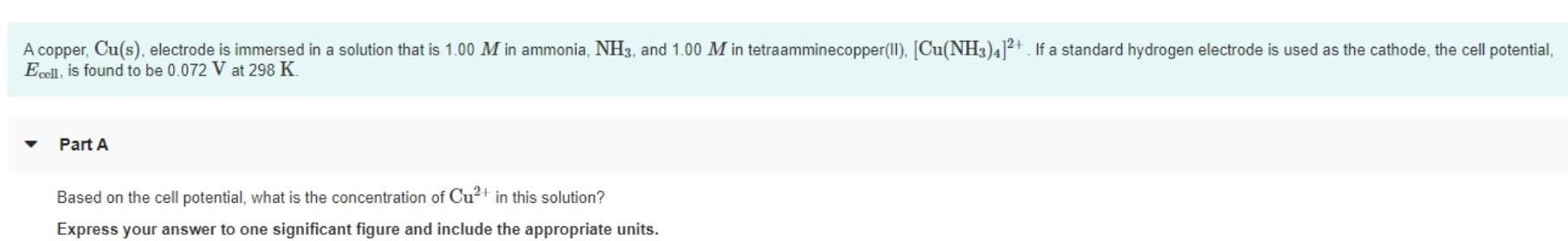
A copper, Cu(s), electrode is immersed in a solution that is 1.00 M in ammonia, NH3, and 1.00 M in tetraamminecopper(II). [Cu(NH3)4]2+ If a standard hydrogen electrode is used as the cathode, the cell potential, Ecell, is found to be 0.072 V at 298 K. Part A Based on the cell potential, what is the concentration of Cu+ in this solution? Express your answer to one significant figure and include the appropriate units.
Step by Step Solution
3.34 Rating (157 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


