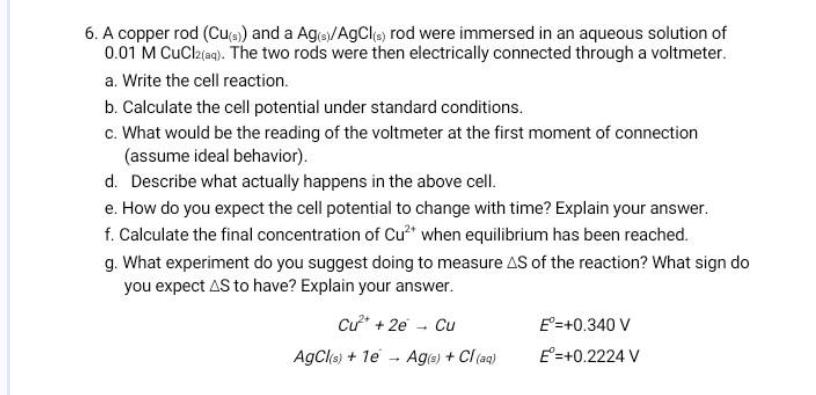
Question: A copper rod ( C u ( s ) ) and a A g ( s ) A g C l ( s ) rod
A copper rod and a rod were immersed in an aqueous solution of The two rods were then electrically connected through a voltmeter.
a Write the cell reaction.
b Calculate the cell potential under standard conditions.
c What would be the reading of the voltmeter at the first moment of connection assume ideal behavior
d Describe what actually happens in the above cell.
e How do you expect the cell potential to change with time? Explain your answer.
f Calculate the final concentration of when equilibrium has been reached.
g What experiment do you suggest doing to measure of the reaction? What sign do you expect to have? Explain your answer.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


