Question: 2) a-) When the iron rod was dipped into 5%CuSO4 solution, indicate the changes that will occur and the related reaction. b-) The zinc rod
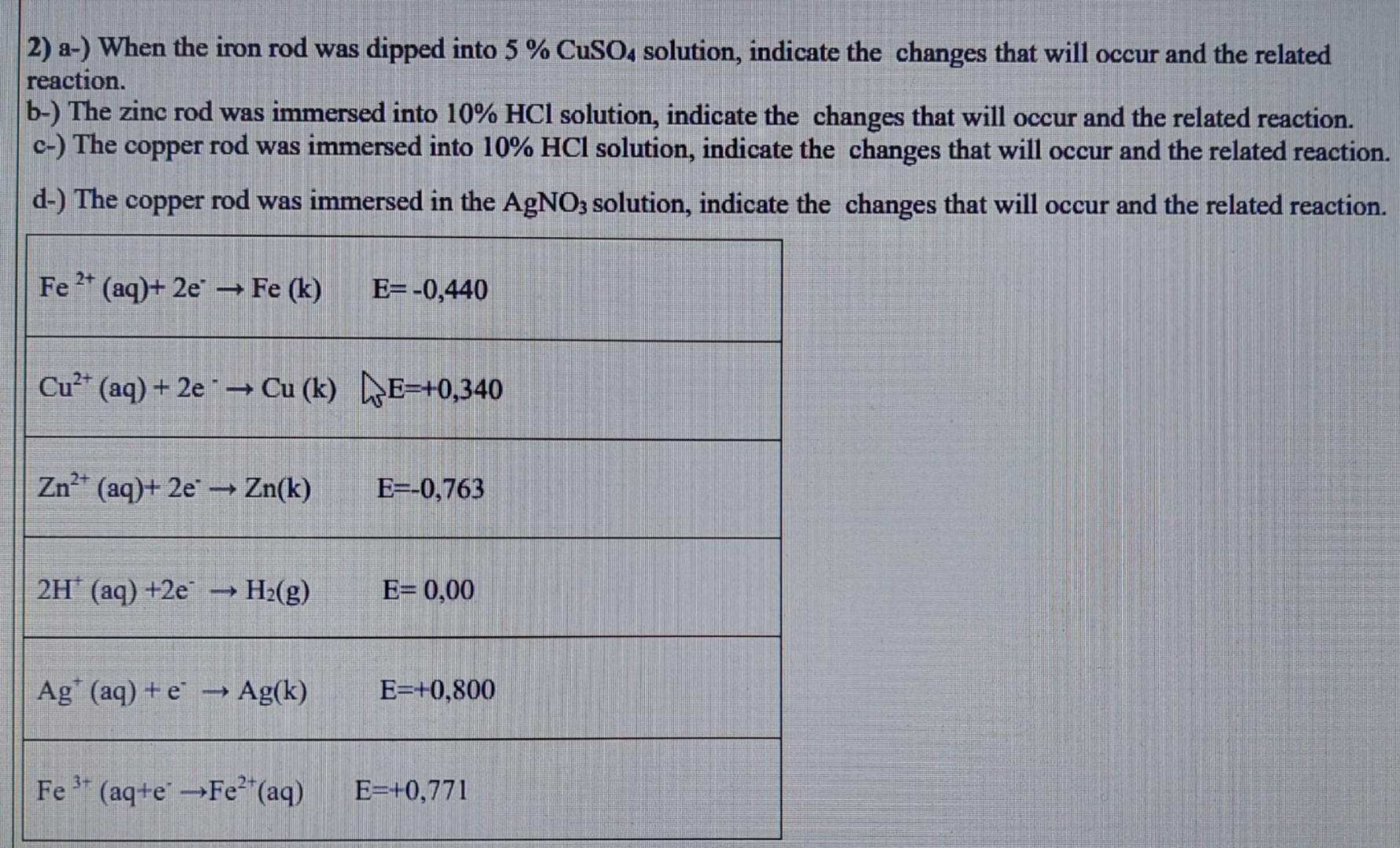
2) a-) When the iron rod was dipped into 5%CuSO4 solution, indicate the changes that will occur and the related reaction. b-) The zinc rod was immersed into 10%HCl solution, indicate the changes that will occur and the related reaction. c-) The copper rod was immersed into 10%HCl solution, indicate the changes that will occur and the related reaction. d-) The copper rod was immersed in the AgNO3 solution, indicate the changes that will occur and the related reaction. \begin{tabular}{|l|l|} \hline Fe2+(aq)+2eFe(k) & E=0,440 \\ Cu2+(aq)+2eCu(k)E=+0,340 \\ \hline Zn2+(aq)+2eZn(k) & E=0,763 \\ \hline H+(aq)+2eH2(g) & E=0,00 \\ \hline Ag+(aq)+eAg(k) & E=+0,800 \\ \hline Fe3+(aq+eFe2+(aq) & E=+0,771 \\ \hline \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


