Question: A CSTR is used for the liquid phase reactions given below under isothermal condition. The CSTR is fed with an aqueous solution of A which
- A CSTR is used for the liquid phase reactions given below under isothermal condition. The CSTR is fed with an aqueous solution of A which initial concentration is 30 mol/dm3. Assume that the ratio of reactor volume to volumetric flow rate, V/v0 = 240 second.
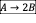
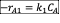
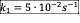
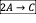
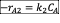

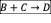
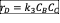
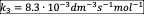
- Write the net rate of reaction of A, B, C.
- Find the concentration of A, B, C, and D at the exit of the reactor.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


