Question: (a) Describe the difference between adsorption by physisorption and adsorption by chemisorption. (b) For the Langmuir isotherm the change in surface coverage, , with time
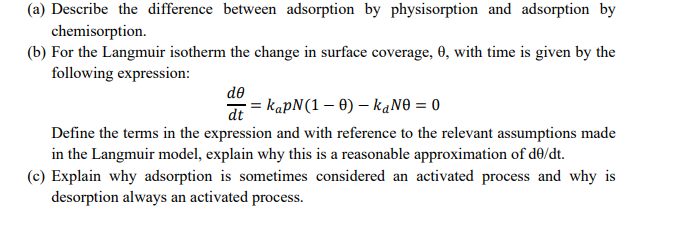
(a) Describe the difference between adsorption by physisorption and adsorption by chemisorption. (b) For the Langmuir isotherm the change in surface coverage, , with time is given by the following expression: dtd=kapN(1)kdN=0 Define the terms in the expression and with reference to the relevant assumptions made in the Langmuir model, explain why this is a reasonable approximation of d/dt. (c) Explain why adsorption is sometimes considered an activated process and why is desorption always an activated process
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


