Question: a ) Develop a model to predict the diffusion - limited flux of arsine to the surface of the wafer. State all your assumptions. b
a Develop a model to predict the diffusionlimited flux of arsine to the surface of the
wafer. State all your assumptions.
b Estimate the initial deposition rate of arsenic in g min onto the surface of a
diameter silicon wafer if
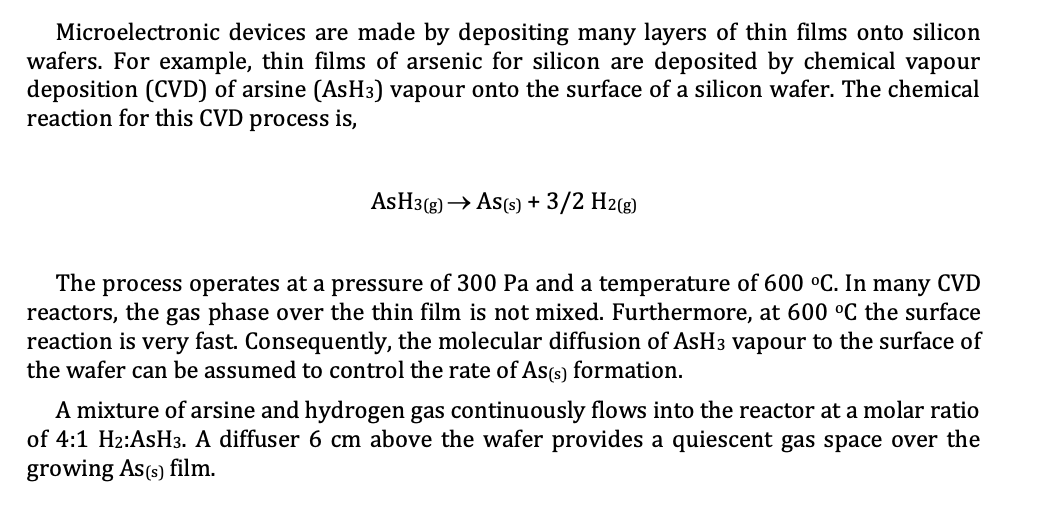
The process operates at a pressure of and a temperature of In many CVD
reactors, the gas phase over the thin film is not mixed. Furthermore, at the surface
reaction is very fast. Consequently, the molecular diffusion of vapour to the surface of
the wafer can be assumed to control the rate of formation.
A mixture of arsine and hydrogen gas continuously flows into the reactor at a molar ratio
of :: A diffuser above the wafer provides a quiescent gas space over the
growing Ass film.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


