Question: a) Develop a process flow diagr (PFD) for following process: CH4+H2OCO+3H2CO+H2OCO2+H2}{T=800KP=600kPa Assume: (H2O:CH4)@ feed =1:5 (the H2O to CH4 mole ratio in the feed is
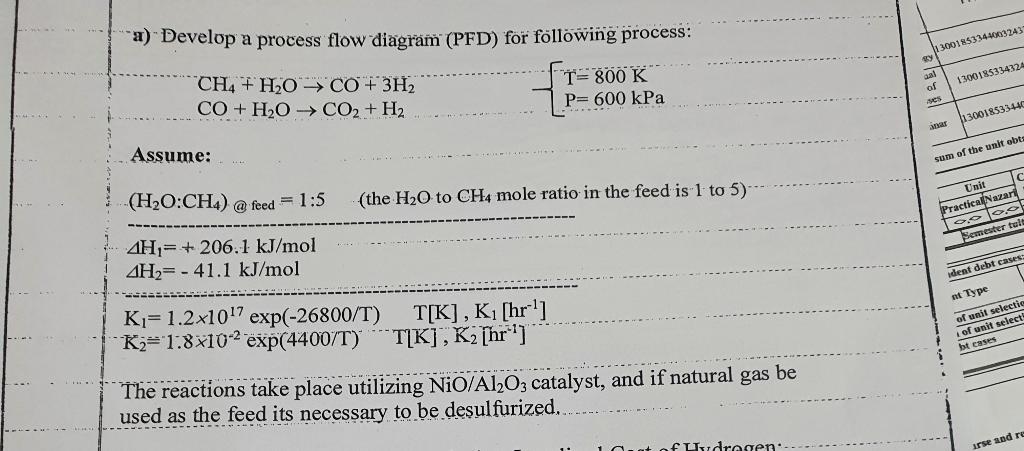
a) Develop a process flow diagr (PFD) for following process: CH4+H2OCO+3H2CO+H2OCO2+H2}{T=800KP=600kPa Assume: (H2O:CH4)@ feed =1:5 (the H2O to CH4 mole ratio in the feed is 1 to 5 ) H1=+206.1kJ/molH2=41.1kJ/molK1=1.21017exp(26800/T)T[K],K1[hr1]K2=1.8102exp(4400/T)T[K],K2[hr1] The reactions take place utilizing NiO/Al2O3 catalyst, and if natural gas be used as the feed its necessary to be desulfurized
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


