Question: A diffusion couple including inert tungsten wire markers was made by plating pure copper onto a block of - brass with x Z n =
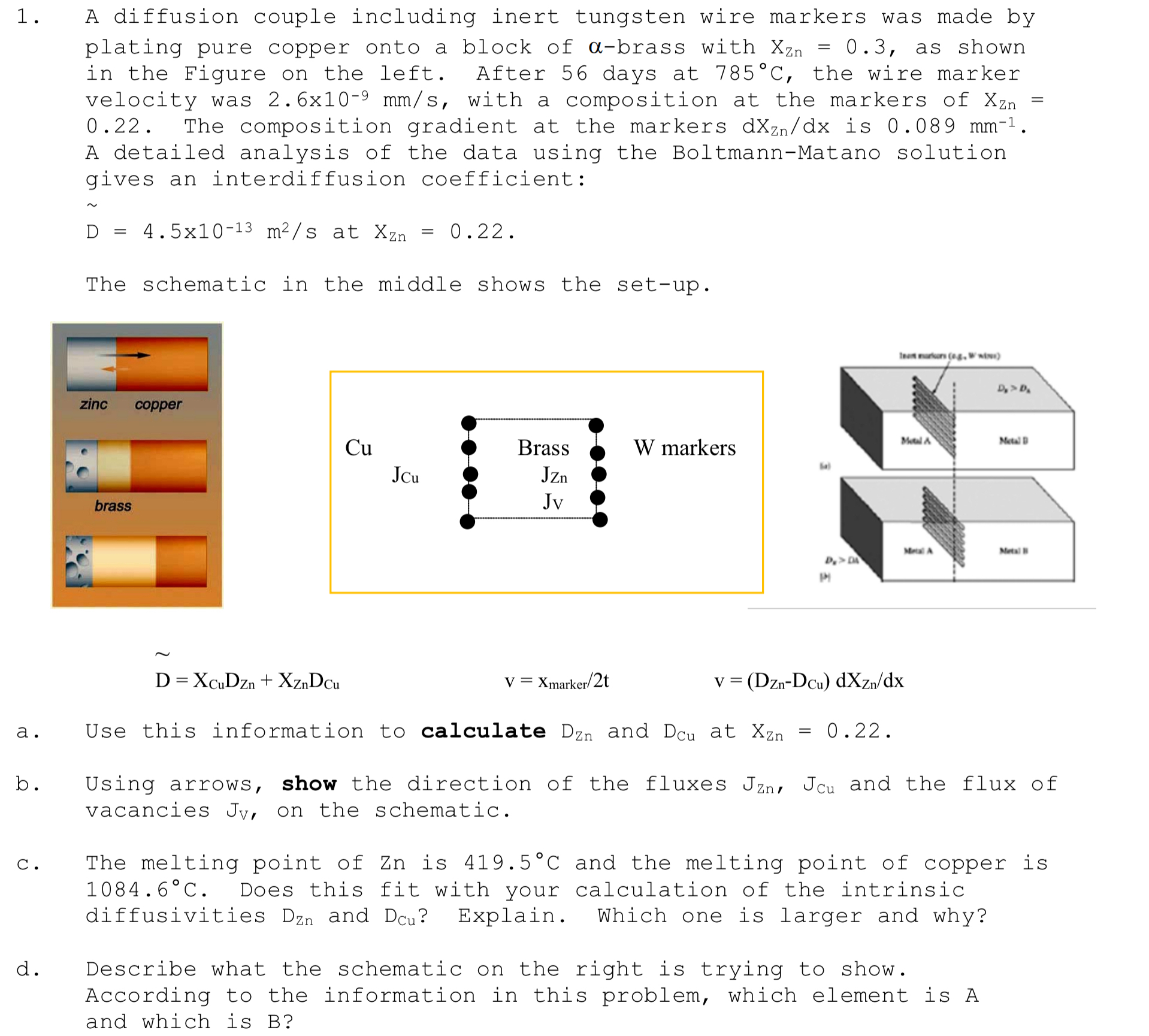
A diffusion couple including inert tungsten wire markers was made by plating pure copper onto a block of brass with as shown in the Figure on the left. After days at the wire marker velocity was with a composition at the markers of The composition gradient at the markers is A detailed analysis of the data using the BoltmannMatano solution gives an interdiffusion coefficient:
at
The schematic in the middle shows the setup
a Use this information to calculate and at
b Using arrows, show the direction of the fluxes and the flux of vacancies on the schematic.
c The melting point of is and the melting point of copper is Does this fit with your calculation of the intrinsic diffusivities and Explain. Which one is larger and why?
d Describe what the schematic on the right is trying to show.
According to the information in this problem, which element is A and which is B
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


