Question: A distillation column separating ethanol from water is shown. Pressure is 1 kg/cm. Instead of having a reboiler, steam (pure water vapor) is injected directly
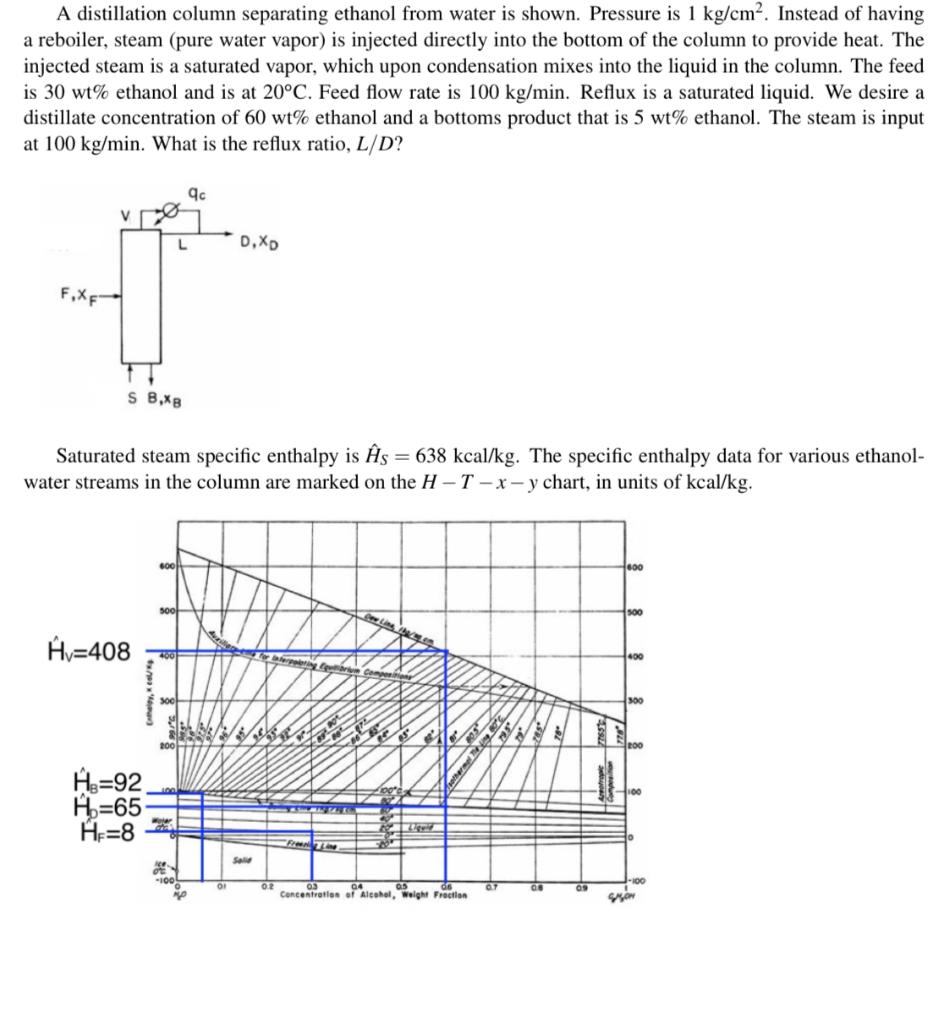
A distillation column separating ethanol from water is shown. Pressure is 1 kg/cm. Instead of having a reboiler, steam (pure water vapor) is injected directly into the bottom of the column to provide heat. The injected steam is a saturated vapor, which upon condensation mixes into the liquid in the column. The feed is 30 wt% ethanol and is at 20C. Feed flow rate is 100 kg/min. Reflux is a saturated liquid. We desire a distillate concentration of 60 wt% ethanol and a bottoms product that is 5 wt% ethanol. The steam is input at 100 kg/min. What is the reflux ratio, L/D? 9c V L D, X F.XF SB,XB Saturated steam specific enthalpy is s = 638 kcal/kg. The specific enthalpy data for various ethanol- water streams in the column are marked on the H-T-x-y chart, in units of kcal/kg. 600 500 Av=408 A3=92 Ho=65 ogs He=8 * 0 OI 02 Concentration of Alcohol, Weight Fraction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


