Question: [ Here is Question 3.D3 as reference: 22.* When water is the more volatile component, we do not need a condenser but can use direct
 [
[
Here is Question 3.D3 as reference:
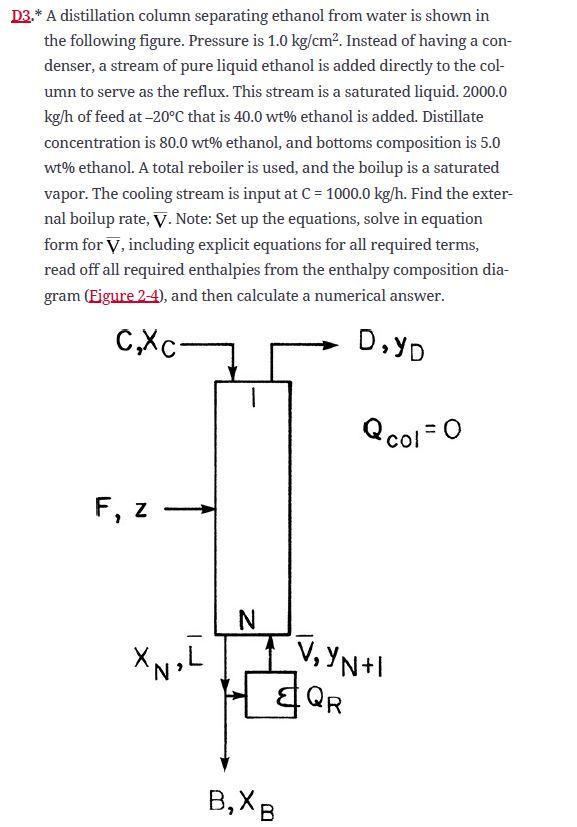
22.* When water is the more volatile component, we do not need a condenser but can use direct cooling with boiling water. This situation was shown in Droblem 3.D3. yD=0.92,xB=0.04,z=0.4 (all mole fractions water), feed is a saturated vapor, feed rate is 1000kmol/h, p=1atm,CMO is valid, the entering cooling water (C) is a saturated liquid and is pure water, and C/D=3/4. Derive and plot the top operating line. Note that external balances (i.e., balances around the entire column) and equilibrium data are not required. D3.* A distillation column separating ethanol from water is shown in the following figure. Pressure is 1.0kg/cm2. Instead of having a condenser, a stream of pure liquid ethanol is added directly to the column to serve as the reflux. This stream is a saturated liquid. 2000.0 kg/h of feed at 20C that is 40.0wt% ethanol is added. Distillate concentration is 80.0wt% ethanol, and bottoms composition is 5.0 wt % ethanol. A total reboiler is used, and the boilup is a saturated vapor. The cooling stream is input at C=1000.0kg/h. Find the external boilup rate, V. Note: Set up the equations, solve in equation form for V, including explicit equations for all required terms, read off all required enthalpies from the enthalpy composition diagram (Eigure 2-4), and then calculate a numerical
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


