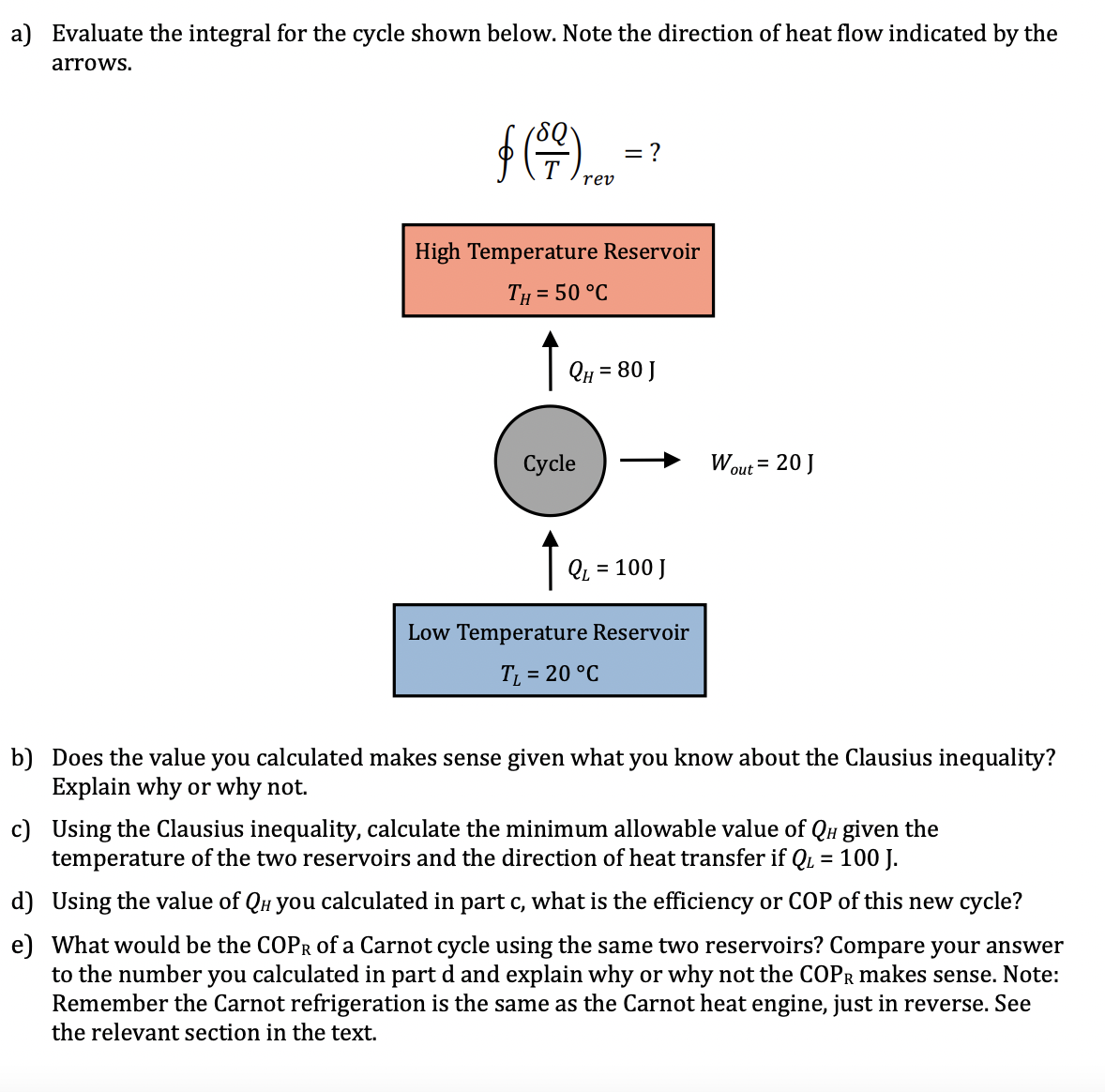
Question: a ) Evaluate the integral for the cycle shown below. Note the direction of heat flow indicated by the arrows. o ( Q T )
a Evaluate the integral for the cycle shown below. Note the direction of heat flow indicated by the
arrows.
Low remperature keservoir
b Does the value you calculated makes sense given what you know about the Clausius inequality?
Explain why or why not.
c Using the Clausius inequality, calculate the minimum allowable value of given the
temperature of the two reservoirs and the direction of heat transfer if
d Using the value of you calculated in part c what is the efficiency or COP of this new cycle?
e What would be the of a Carnot cycle using the same two reservoirs? Compare your answer
to the number you calculated in part and explain why or why not the makes sense. Note:
Remember the Carnot refrigeration is the same as the Carnot heat engine, just in reverse.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


