Question: (a) Explain what is meant by the terms diffusion-control and activation-control in terms of reaction kinetics. [3 marks) (b) The decomposition of N2O5 with the
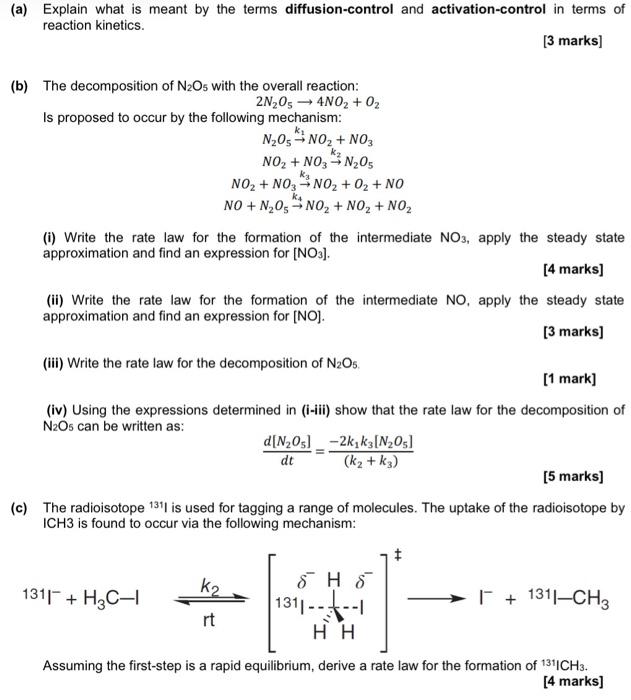
(a) Explain what is meant by the terms diffusion-control and activation-control in terms of reaction kinetics. [3 marks) (b) The decomposition of N2O5 with the overall reaction: 2N2054NO2 + O2 Is proposed to occur by the following mechanism: N203 NO2 + NO3 NO2 + NO3-N,Os NO2 + NO, NO2 + O2 + NO NO + N20 - NO2 + NO2 + NO2 (i) Write the rate law for the formation of the intermediate NO3, apply the steady state approximation and find an expression for [NO3]. [4 marks) (ii) Write the rate law for the formation of the intermediate NO, apply the steady state approximation and find an expression for (NO). [3 marks] (ii) Write the rate law for the decomposition of N206 [1 mark] (iv) Using the expressions determined in (i-iii) show that the rate law for the decomposition of N2Os can be written as: d[N,05) -2k, k3(N2O) dt (kz + k) [5 marks] (c) The radioisotope 1311 is used for tagging a range of molecules. The uptake of the radioisotope by ICH3 is found to occur via the following mechanism: k2 1311+ H2C 1311 ------ HH + 131-CH3 rt Assuming the first step is a rapid equilibrium, derive a rate law for the formation of 1311CH3. [4 marks]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


