Question: a. For a 30% mixture that starts out subcooled and then is heated, at what temperature will begin to form vapor (bubbles)? What is

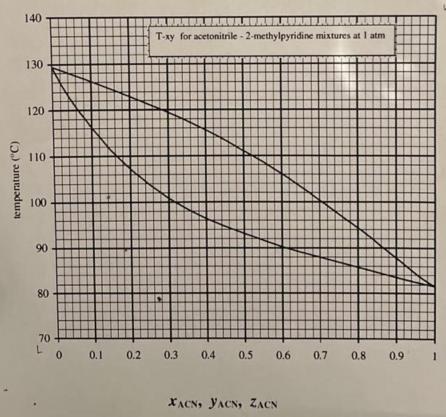
a. For a 30% mixture that starts out subcooled and then is heated, at what temperature will begin to form vapor (bubbles)? What is the composition of the first bubbles formed? b. For a 30% mixture that starts out superheated and then is cooled, at what temperature will begin to form liquid (dew drops)? What is the composition of the first condensate formed? c. For a 30% mixture at 225F, what fraction of the mixture is vapor, and what fraction is in the form of liquid? What are the liquid and vapor phase compositions? d. At what temperature will the vapor fraction be 50% (a 1:1 vapor to liquid ratio)? temperature (C) 140 130 120 110 100 90 80 T-xy for acetonitrile-2-methylpyridine mixtures at I atm L 71 70+ 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1 XACN, YACN, ZACN
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


