Question: a) For A B, write the rate equations for the zero, first and second order reactions. b) Write the half-life period (to 0,5) (expression) for
a) For A B, write the rate equations for the zero, first and second order reactions.
b) Write the half-life period (to 0,5) (expression) for the first order reaction (for A-B).
(Half-life (symbol t1/2) is the time required for a quantity to reduce to half of its initial value)
For the reaction A B the following experimental data were obtained. Determine the order of the reaction and rate constant. CA0 = 10 mole/L
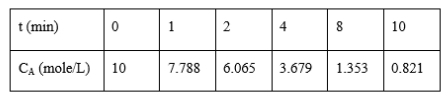
\begin{tabular}{|l|l|l|l|l|l|l|} \hline t(min) & 0 & 1 & 2 & 4 & 8 & 10 \\ \hline CA(mole/L) & 10 & 7.788 & 6.065 & 3.679 & 1.353 & 0.821 \\ \hline \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


