Question: a. For a binary mixture, =6x1x2, where is some molar property of the mixture and xi is the mole fraction of component i. Derive an
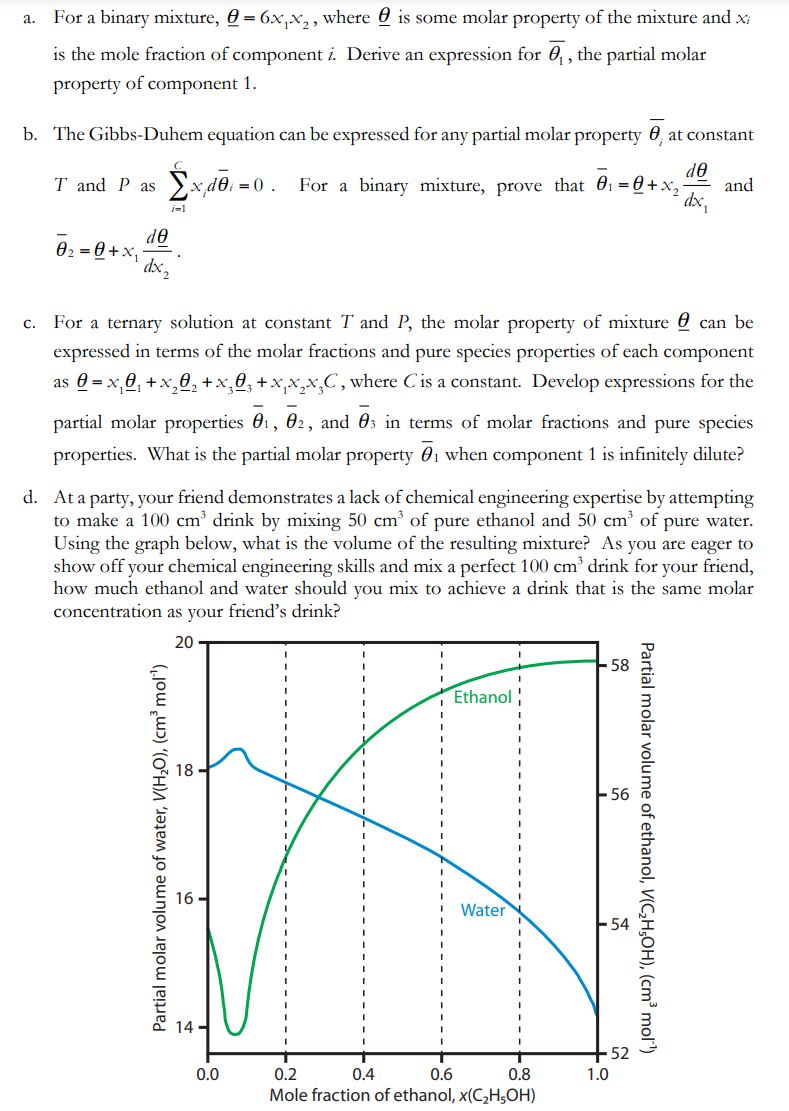
a. For a binary mixture, =6x1x2, where is some molar property of the mixture and xi is the mole fraction of component i. Derive an expression for 1, the partial molar property of component 1. b. The Gibbs-Duhem equation can be expressed for any partial molar property i at constant T and P as i=1Cxidi=0. For a binary mixture, prove that 1=+x2dx1d and 2=+x1dx2d c. For a ternary solution at constant T and P, the molar property of mixture can be expressed in terms of the molar fractions and pure species properties of each component as =x11+x22+x33+x1x2x3C, where C is a constant. Develop expressions for the partial molar properties 1,2, and 3 in terms of molar fractions and pure species properties. What is the partial molar property 1 when component 1 is infinitely dilute? d. At a party, your friend demonstrates a lack of chemical engineering expertise by attempting to make a 100cm3 drink by mixing 50cm3 of pure ethanol and 50cm3 of pure water. Using the graph below, what is the volume of the resulting mixture? As you are eager to show off your chemical engineering skills and mix a perfect 100cm3 drink for your friend, how much ethanol and water should you mix to achieve a drink that is the same molar concentration as your friend's drink
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


