Question: A galvanic cell which operates at 25C consists on the based two standard half-reactions. The half-cells are connected by a wire for electron movement and
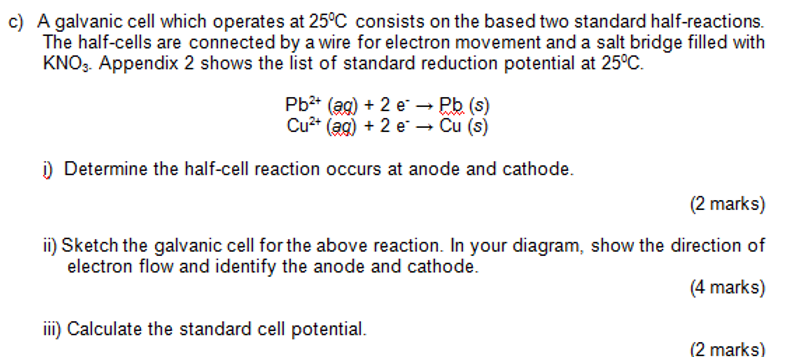
A galvanic cell which operates at 25C consists on the based two standard half-reactions. The half-cells are connected by a wire for electron movement and a salt bridge filled with KNO3. Appendix 2 shows the list of standard reduction potential at 25C. Pb2+(ag)+2ePb(s)Cu2+(ag)+2eCu(s) i) Determine the half-cell reaction occurs at anode and cathode. (2 marks) ii) Sketch the galvanic cell for the above reaction. In your diagram, show the direction of electron flow and identify the anode and cathode. (4 marks) iii) Calculate the standard cell potential
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


