Question: A gas containing 60 mol % CH4, 20 mol % CH6 ,5 mol % CO and 5 mol % O and the rest N
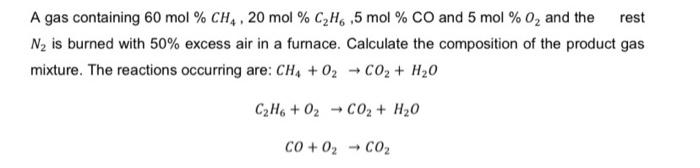
A gas containing 60 mol % CH4, 20 mol % CH6 ,5 mol % CO and 5 mol % O and the rest N is burned with 50% excess air in a furnace. Calculate the composition of the product gas mixture. The reactions occurring are: CH4 + O CO + HO CzH+0z - CO, + H,0 CO+O CO -> ->
Step by Step Solution
There are 3 Steps involved in it
To calculate the composition of the product gas mixture we need to perform a combustion analysis Well follow these steps 1 Convert the mole percentage... View full answer

Get step-by-step solutions from verified subject matter experts


