Question: 1. Using data in Appendix C, calculate AH, AS', and AG at 298K for each of the following reactions. Indicate whether the reaction is

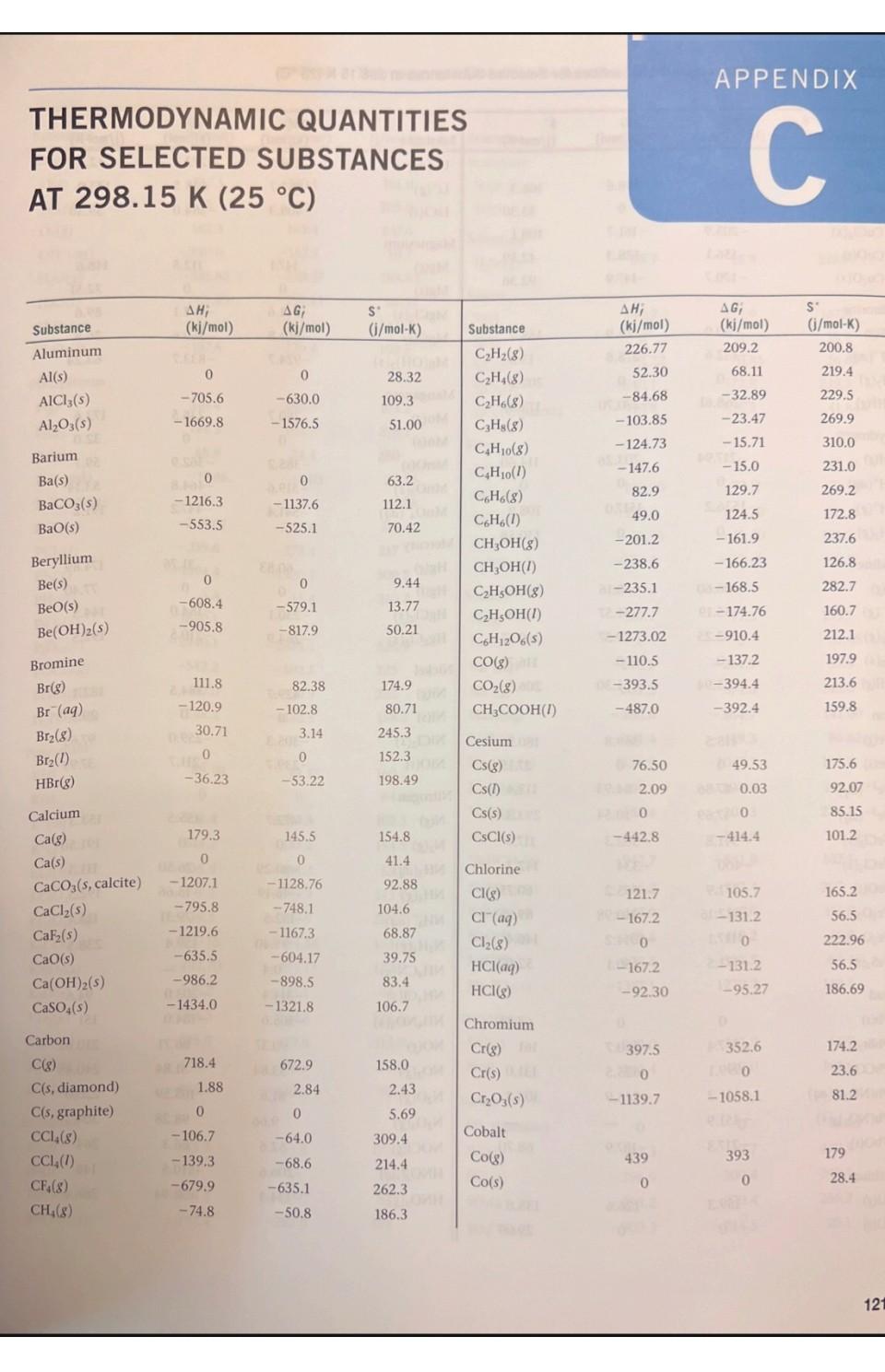
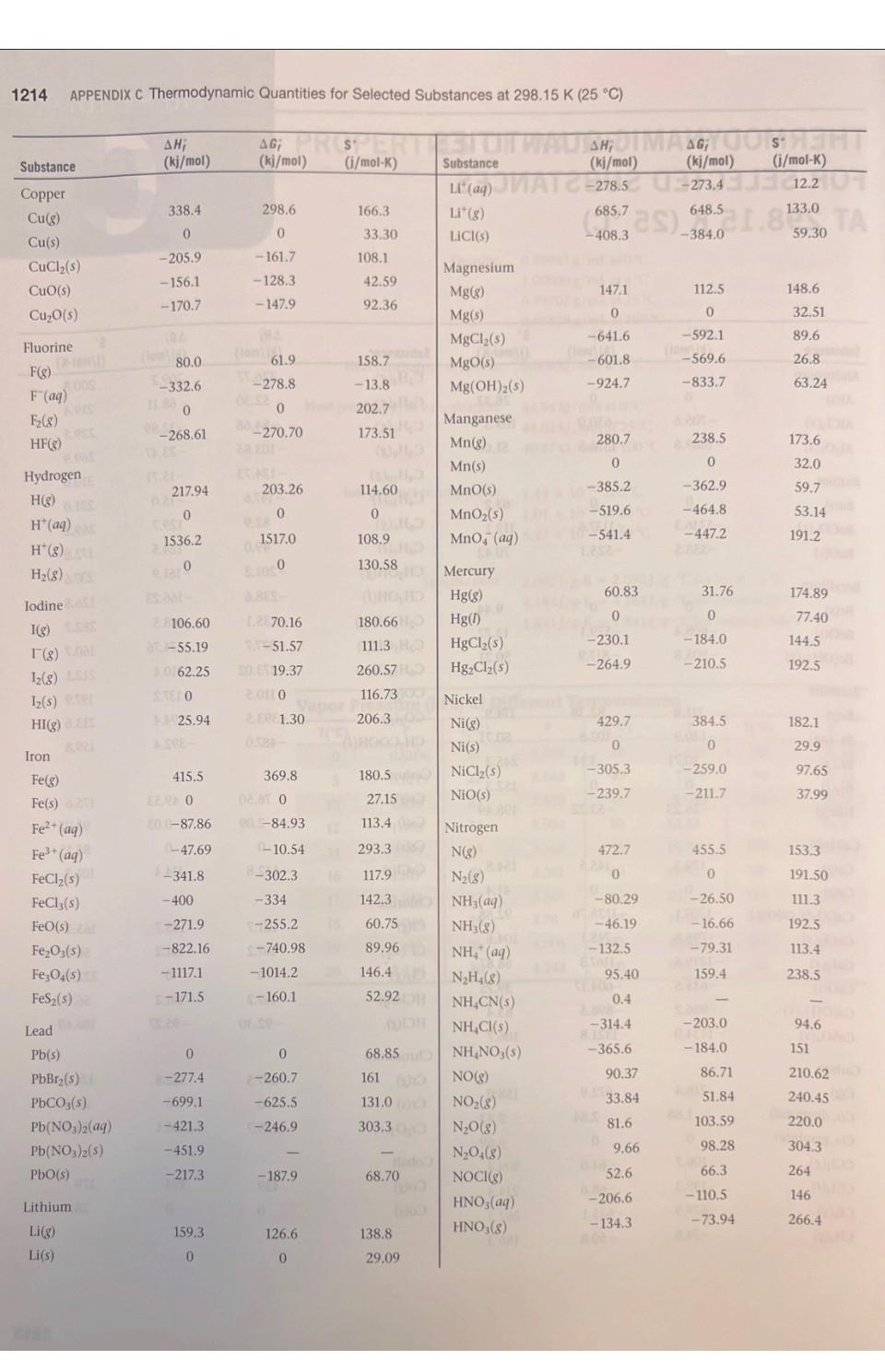
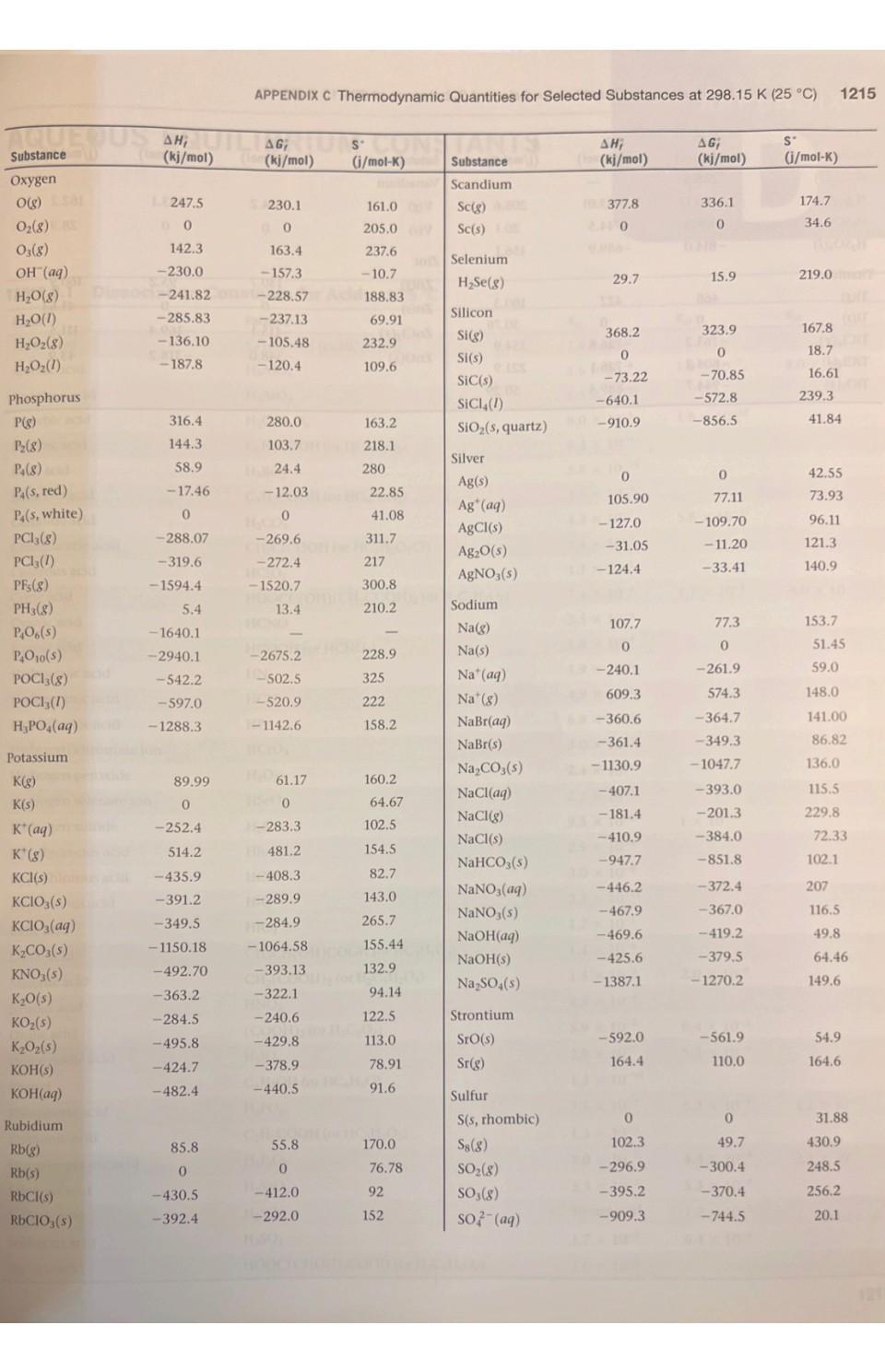
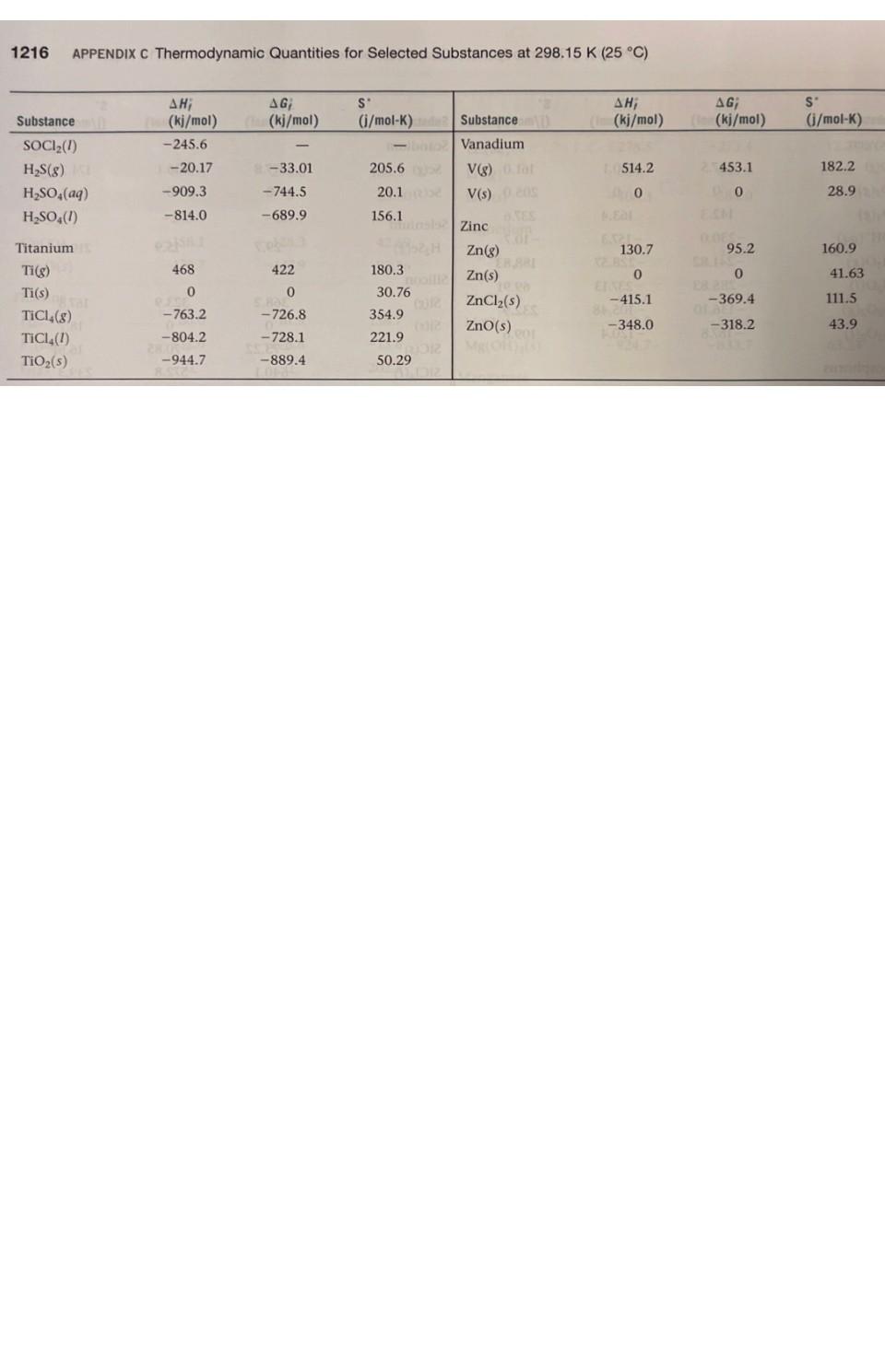
1. Using data in Appendix C, calculate AH, AS', and AG at 298K for each of the following reactions. Indicate whether the reaction is spontaneous at 298K under standard conditions. (a) Ti (s) + 2 Cl(g) TiCl4 (1) (b) HCI (aq) + NaOH (aq) NaCl (aq) + HO (1) (c) CF4 (g) + 2 H (g) C(s) + 4 HF (g) (d) NaCO3 (s) + 2 HNO3(aq) 2 NaNO3(aq) + CO (g) + HO (1) THERMODYNAMIC QUANTITIES FOR SELECTED SUBSTANCES AT 298.15 K (25 C) Substance Aluminum Al(s) AICI3(s) AlO3(s) Barium Ba(s) BaCO3(s) BaO(s) Beryllium Be(s) BeO(s) Be (OH)2 (s) Bromine Br(g) Br (aq) Br(8) Br(1) HBr(g) Calcium Ca(g) Ca(s) CaCO3(s, calcite) CaCl(s) CaF(s) CaO(s) Ca(OH)2(s) CaSO4(s) Carbon C(g) C(s, diamond) C(s, graphite) CC14(8) CC1 (1) CF4(8) CH(8) AH; (kj/mol) 0 -705.6 -1669.8 0 -1216.3 -553.5 0 -608.4 -905.8 111.8 - 120.9 30.71 -36.23 179.3 0 -1207.1 -795.8 -1219.6 -635.5 -986.2 -1434.0 9718.4 1.88 0 -106.7 -139.3 -679.9 -74.8 AG; (kj/mol) 0 -630.0 -1576.5 0 - 1137.6 -525.1 0 -579.1 -817.9 82.38 - 102.8 3.14 0 -53.22 145.5 0 -1128.76 -748.1 -1167.3 -604.17 -898.5 -1321.8 672.9 2.84 0 -64.0 -68.6 -635.1 -50.8 S (j/mol-K) 28.32 109.3 51.00 63.2 112.1 70.42 9.44 13.77 50.21 174.9 80.71 6H 245.3 152.3 198.49 154.8 41.4 92.88 104.6 68.87 39.75 83.4 106.7 158.0 309.4 214.4 262.3 186.3 2.43 5.69 Will Substance CH(8) CH4(8) CH6(8) C3H8(8) C4H10(8) C4H10(1) C6H6(8) C6H6(1) CHOH(g) CHOH(1) CH5OH (8) CHOH(1) C6H12O6(S) CO(g) CO(8) CH3COOH(1) Cesium08 Cs(g) Cs(1) Cs(s) ELLIPS CsCl(s) Chlorine CI(g) HW CI (aq) Cl(8) 11.8 HCl(aq) HCI(g) Chromium Cr(g) Cr(s) E CrO3(s) Cobalt Co(g) Co(s) B AH; (kj/mol) 226.77 52.30 -84.68 -103.85 -124.73 -147.6 82.9 49.0 -201.2 -238.6 al-235.1 -277.7 -1273.02 -110.5 06-393.5 -487.0 76.50 2.09 1200 -442.8 121.7 SHOO 1-167.2 -92.30 397.5 22000 -1139.7 APPENDIX C 439 0 AG; (kj/mol) 209.2 68.11 -32.89 -23.47 -15.71 -15.0 129.7 124.5 -161.9 -166.23 08-168.5 1-174.76 25-910.4 -137.2 40-394.4 -392.4 8-167.2-131.2 49.53 08 0.03 eat200 -414.4 105.7 150 2-131.2 -95.27 D 352.6 LABO -1058.1 EXIDE 393 0 S' (j/mol-K) 200.8 219.4 229.5 269.9 310.0 231.0 269.2 172.8 237.6 126.8 282.7 160.7 212.1 197.9 213.6 159.8 175.6 92.07 85.15 101.2 165.2 56.5 222.96 56.5 186.69 174.2 23.6 81.2 179 28.4 121 1214 APPENDIX C Thermodynamic Quantities for Selected Substances at 298.15 K (25 C) Substance Copper Cu(g) Cu(s) CuCl(s) CuO(s) CuO(s) Fluorine F(g) F (aq) F(8) HF(g) Hydrogen H(g) H*(aq) H*(8) H(8) ES lodine 051 I(g) 58 (8) 00 12(8) LESS 1(s) e HI(g) E Iron Fe(g) Fe(s) 21 Fe+ (aq) Fe+ (aq) FeCl(s) FeCl3(s) FeO(s) zal FeO3(s) Fe3O4(s) FeS(s) Lead Pb(s) PbBr(S) PbCO3(s) 2 Pb(NO3)2(aq) Pb(NO3)2(S) PbO(s) Lithium Li(g) Li(s) AH; (kj/mol) 338.4 0 -205.9 -156.1 -170.7 80.0 -332.6 0 -268.61 217.94 0 1536.2 0 28106.60 8-55.19 A0162.25 STEL O 25.94 415.5 EERO 20.0-87.86 (-47.69 -341.8 -400 -271.9 -822.16 -1117.1 1-171.5 0 -277.4 -699.1 -421.3 -451.9 -217.3 159.3 0 (fo AG; PROSPERT (kj/mol) (i/mol-K) 298.6 0 -161.7 -128.3 -147.9 61.9 -278.8 0 88-270.70 203.26 0 1517.0 8.1000 Le 70.16 1.-51.57 50. 19.37 20110 ERE 1.30 369.8 02.81 0 POL-84.93 (-10.54 8-302.3 -334 -255.2 -740.98 -1014.2 -160.1 0 2-260.7 -625.5 -246.9 -187.9 126.6 0 166.3 33.30 108.1 42.59 92.36 158.7 -13.8 202.7 173.51 114.60 0 108.9 130.58 1000 HD 180.66 111.3 HO 260.57 H 116.737 206.30 OID 180.5) 27.15 113.4 293.3 117.9 142.3 del 60.75 89.96 146.45 52.92 68.85 161 ( 131.0 303.300 68.70 Hid 138.8 DH 29.09 STUIT WAUTO; DI MAGYOOSHEHT Substance (kj/mol) (kj/mol) (i/mol-K) LI (aq)MATC-278.5-273.4 12.20 Li* (g) LICI(s) Magnesium Mg(g) Mg(s) MgCl(s) MgO(s) Mg(OH)(s) Manganese Mn(g) Mn(s) MnO(s) MnO (s) MnO4 (aq) Mercury Hg(g) Hg(1) HgCl(s) HgCl (s) Nickel Ni(g) Ni(s) NiCl(s) NIO(s) Nitrogen N(g) N (8) NH3(aq) NH3(g) NH, (aq) NH(8) NHCN(S) NHCl(s) NHNO3(s) NO(g) NO(8) NO(g) NO4(8) NOCI(g) HNO3(aq) HNO3 (8) 686725) 648.51.833,00 TA -408.3 147.1 0 -641.6 -601.8 -924.7 280.7 0 -385.2 -519.6 -541.4 60.83 0 -230.1 -264.9 429.7 0 -305.3 -239.7 472.7 0 -80.29 -46.19 -132.5 42 Eta 95.40 0.4 -314.4 8.150.0 -365.6 90.37 33.84 81.6 9.66 52.6 -206.6 -134.3 (lo 112.5 0 -592.1 -569.6 -833.7 238.5 0 -362.9 -464.8 -447.2 31.76 0 -184.0 -210.5 384.5 0 -259.0 -211.7 455.5 0 -26.50 -16.66 -79.31 159.4 -203.0 -184.0 86.71 51.84 103.59 98.28 66.3 -110.5 -73.94 148.6 32.51 89.6 26.8 63.24 173.6 32.0 59.7 53.14 191.2 174.89 77.40 144.5 192.5 182.1 29.9 97.65 37.99 153.3 191.50 111.3 192.5 113.4 238.5 94.6 151 210.620 240.45 220.0 304.3 264 146 266.4 Substance Oxygen O(g) 0(8) 03(8) OH (aq) HO(g) HO(1) HO(8) HO(1) Phosphorus P(g) P(8) P4(8) P(s, red) P,(s, white) PC13 (8) PC13 (1) PFs (8) PH3(g) P406(S) PO10(S) POCI3 (8) POCI (1) HPO(aq) Potassium K(g) K(s) K* (aq) K*(8) KCI(s) KCIO3(s) KCIO3(aq) KCO3(s) KNO3(s) KO(s) KO (s) KO (s) KOH(s) KOH(aq) Rubidium Rb(g) Rb(s) RbCl(s) RbCIO3(s) JUS AHUI (kj/mol) 1247.5 0 142.3 -230.0 -285.83 -136.10 -187.8 316.4 144.3 58.9 -17.46 0 -241.82-228.57 -288.07 -319.6 -1594.4 5.4 -1640.1 -2940.1 -542.2 -597.0 -1288.3 89.99 0 -252.4 514.2 -435.9 -391.2 -349.5 -1150.18 -492.70 -363.2 -284.5 -495.8 -424.7 -482.4 APPENDIX C Thermodynamic Quantities for Selected Substances at 298.15 K (25 C) 85.8 0 -430.5 -392.4 AG; SU (kj/mol) (i/mol-K) 230.1 0 163.4 -157.3 -237.13 -105.48 -120.4 280.0 103.7 24.4 -12.03 0 -269.6 -272.4 -1520.7 13.4 61.17 0 -283.3 481.2 161.0 205.0 237.6 -10.7 H-408.3 -289.9 -284.9 -1064.58 -393.13 -322.1 -240.6 -429.8 -378.9. -440.5 Conical 188.83 69.91 232.9 109.6 55.8 0 -412.0 -292.0 163.2 218.1 280 -2675.2 228.9 11-502.5 325 H-520.9 222 1-1142.6 158.2 22.85 41.08 311.7 217 300.8 210.2 160.2 64.67 102.5 154.5 82.7 143.0 265.7 155.44 132.9 94.14 122.5 113.0 78.91 91.6 CHETICALO 170.0 76.78 92 152 Substance Scandium Sc(g) Sc(s) and Selenium HSe(g) Silicon Si(g) Si(s) SIC(s) SIC14(1) SiO (s, quartz) Silver Ag(s) Ag* (aq) AgCl(s) AgO(s) AgNO3(s) Sodium Na(g) Na(s) Na* (aq) Na' (g) NaBr(aq) NaBr(s) NaCO3(s) NaCl(aq) NaCl(g) NaCl(s) NaHCO3(s) NaNO3(aq) NaNO3(s) NaOH(aq) NaOH(s) NaSO4(s) Strontium Sro(s) Sr(g) Sulfur S(s, rhombic) S8(8) SO(8) SO3(8) SO2 (aq) , (kj/mol) 377.8 200 29.7 368.2 0 -73.22 -640.1 1-910.9 0 105.90 -127.0 -31.05 -124.4 107.7 0 -240.1 609.3 -360.6 -361.4 -1130.9 -407.1 -181.4 -410.9 -947.7 -446.2 -467.9 -469.6 -425.6 -1387.1 -592.0 164.4 0 102.3 -296.9 -395.2 -909.3 AG; (kj/mol) 336.1 0 15.9 323.9 0 -70.85 -572.8 -856.5 0 77.11 -109.70 -11.20 -33.41 77.3 0 -261.9 574.3 -364.7 -349.3 -1047.7 -393.0 -201.3 -384.0 -851.8 -372.4 -367.0 -419.2 -379.5 -1270.2 -561.9 110.0 0 49.7 -300.4 -370.4 -744.5 S' (i/mol-K) 174.7 34.6 219.0 167.8 18.7 16.61 239.3 41.84 42.55 73.93 96.11 121.3 140.9 153.7 51.45 59.0 148.0 141.00 86.82 136.0 115.5 229.8 1215 72.33 102.1 207 116.5 49.8 64.46 149.6 54.9 164.6 31.88 430.9 248.5 256.2 20.1 1216 APPENDIX C Thermodynamic Quantities for Selected Substances at 298.15 K (25 C) Substance SOCI(1) HS(8) HSO4(aq) HSO4(1) Titanium Ti(g) Ti(s) TiCl (8) TICI (1) TiO (s) AH; AG (kj/mol) Cha(kj/mol) -245.6 -20.17 -909.3 -814.0 468 0 -763.2 -804.2 -944.7 - -33.01 -744.5 -689.9 422 0 -726.8 -728.1 -889.4 S (i/mol-K)de -bto 205.6 20.1 156.1 180.3 insis nooill2 30.76 354.9 221.9 50.29 ALDIZ Substance Vanadium V(g) 0.fat V(s) 0.205 Zinc Zn(g) Zn(s) ZnCl(s) ZnO(s) 10.08 201 AH; (m(kj/mol) 1514.2 0 130.7 0 -415.1 -348.0 X2 BST AG; ((kj/mol) 25453.1 00 0.065 95.2 0 -369.4 -318.2 S' (j/mol-K) 182.2 28.9 160.9 41.63 111.5 43.9
Step by Step Solution
3.50 Rating (150 Votes )
There are 3 Steps involved in it
Sure we can use the data provided in Appendix C to calculate the standard enthalpy change H the standard entropy change S and the standard Gibbs free ... View full answer

Get step-by-step solutions from verified subject matter experts


