Question: A gas mixture containing methane (1), nitrogen (2), and carbon dioxide (3) (60mol% methane, 10 mol% nitrogen and 30 mol% carbon dioxide) is in contact
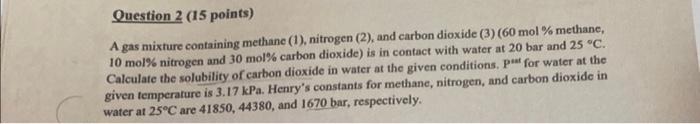
A gas mixture containing methane (1), nitrogen (2), and carbon dioxide (3) (60mol% methane, 10 mol\% nitrogen and 30 mol\% carbon dioxide) is in contact with water at 20 bar and 25C. Calculate the solubility of carbon dioxide in water at the given conditions. Puat for water at the given temperature is 3.17kPa. Henry's constants for methane, nitrogen, and carbon dioxide in water at 25C are 41850,44380 , and 1670 bar, respectively
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


