Question: Question #2 (8 points) Oxygen is diffusing through a stagnant gas mixture containing 50% methane, 30% hydrogen and 20% carbon dioxide by volume. The total
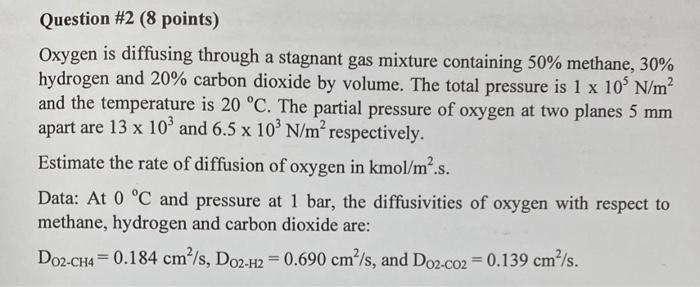
Question #2 (8 points) Oxygen is diffusing through a stagnant gas mixture containing 50% methane, 30% hydrogen and 20% carbon dioxide by volume. The total pressure is 1 x 10 N/m and the temperature is 20 C. The partial pressure of oxygen at two planes 5 mm apart are 13 x 10' and 6.5 x 10' N/m respectively. Estimate the rate of diffusion of oxygen in kmol/m.s. Data: At 0 C and pressure at 1 bar, the diffusivities of oxygen with respect to methane, hydrogen and carbon dioxide are: Do2-CH4 = 0.184 cm/s, Do2-H2 = 0.690 cm /s, and D02-co2 = 0.139 cm/s. =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


