Question: A gas stream is 9 0 mol % N 2 and 1 0 mol % C O 2 . We wish to absorb C O
A gas stream is mol and mol We wish to absorb into water. The inlet water
is pure and is at Operation is isothermal and is at atm. If the liquid flow rate is times
the minimum liquid flow rate, how many equilibrium stages are required to absorb of the
Choose a basis of of entering gas.
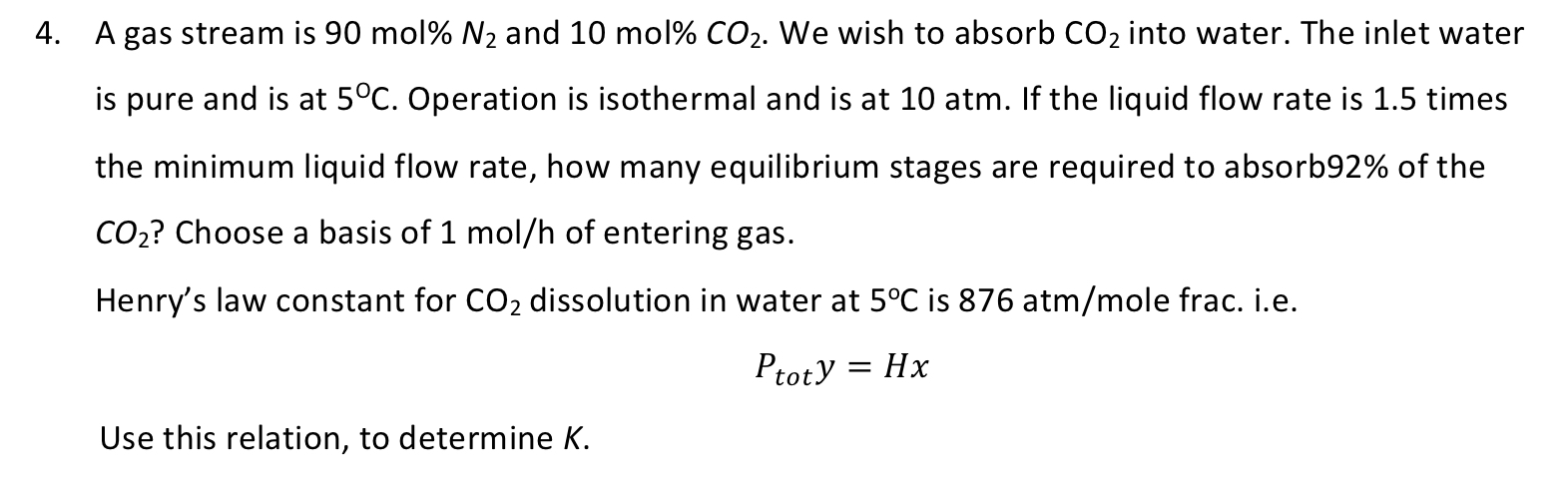
Henry's law constant for dissolution in water at is ole frac. ie
Use this relation, to determine
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


