Question: Assignment #2 (14 questions) A compound was found to contain 54.53% carbon, 9.15% hydrogen, and 36.32% oxygen. Determine the enpirical formula for this compound by
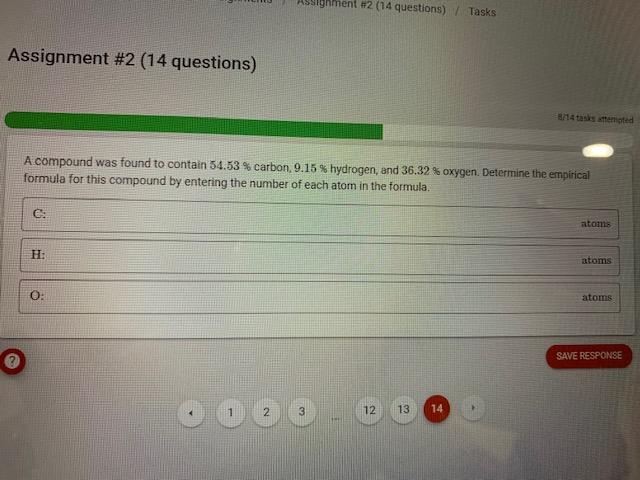
Assignment \#2 (14 questions) A compound was found to contain 54.53% carbon, 9.15% hydrogen, and 36.32% oxygen. Determine the enpirical formula for this compound by entering the number of each atom in the formula
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


