Question: A gas stream with 76 mol % AICI (g), 16 mol % AlCl3(g), and 8 mol % He(g), flowing at a rate of 0.96
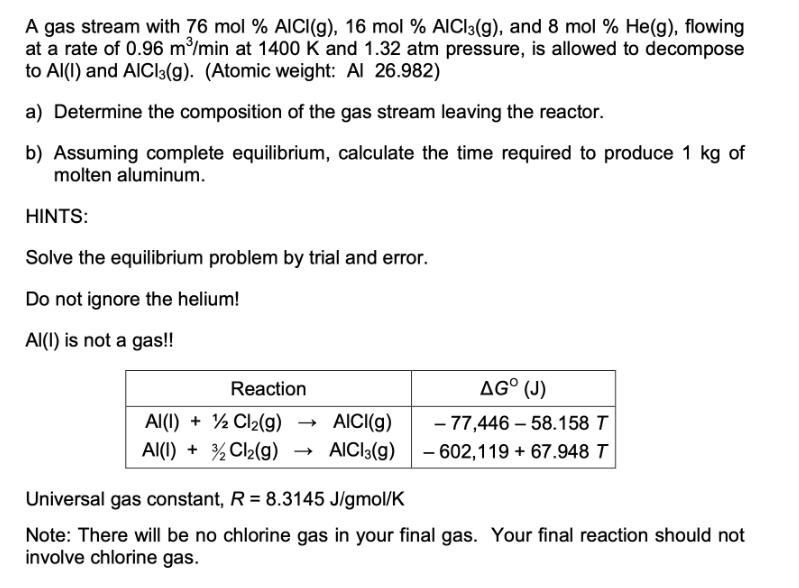
A gas stream with 76 mol % AICI (g), 16 mol % AlCl3(g), and 8 mol % He(g), flowing at a rate of 0.96 m/min at 1400 K and 1.32 atm pressure, is allowed to decompose to Al(I) and AlCl3(g). (Atomic weight: Al 26.982) a) Determine the composition of the gas stream leaving the reactor. b) Assuming complete equilibrium, calculate the time required to produce 1 kg of molten aluminum. HINTS: Solve the equilibrium problem by trial and error. Do not ignore the helium! Al() is not a gas!! Reaction AI(I) + 2Cl(g) AICI(g) AI(I) + 2Cl(g) >> AICI3(g) AG (J) -77,446-58.158 T - 602,119 +67.948 T Universal gas constant, R = 8.3145 J/gmol/K Note: There will be no chlorine gas in your final gas. Your final reaction should not involve chlorine gas.
Step by Step Solution
There are 3 Steps involved in it
Problem Solving a Determining the Composition of the Leaving Stream This problem requires using tria... View full answer

Get step-by-step solutions from verified subject matter experts


