Question: A generic solid, X, has a molar mass of 71.6 g/mol. In a constant-pressure calorimeter, 11.6 g of X is dissolved in 295 g of
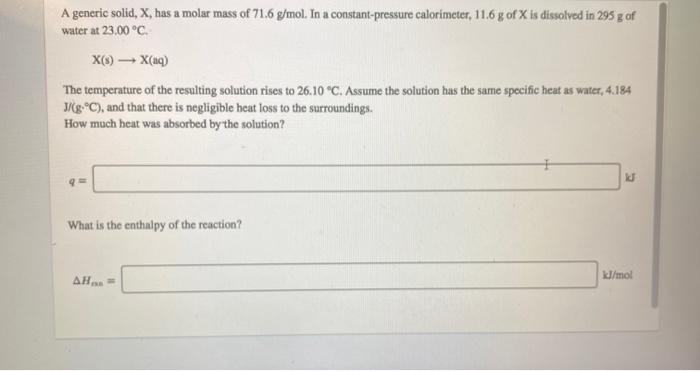
A generic solid, X, has a molar mass of 71.6 g/mol. In a constant-pressure calorimeter, 11.6 g of X is dissolved in 295 g of water at 23,00 C X(9) X(4) The temperature of the resulting solution rises to 26.10 C. Assume the solution has the same specific heat as water, 4.184 J/(g:C), and that there is negligible heat loss to the surroundings. How much heat was absorbed by the solution? IJ What is the enthalpy of the reaction? AH. kl/mol
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


