Question: HO Alexanda EM 1212 (Sections D, E and J) - Spring 21 - HURST > Activities and Due Dates > HW 10 50% Resources I
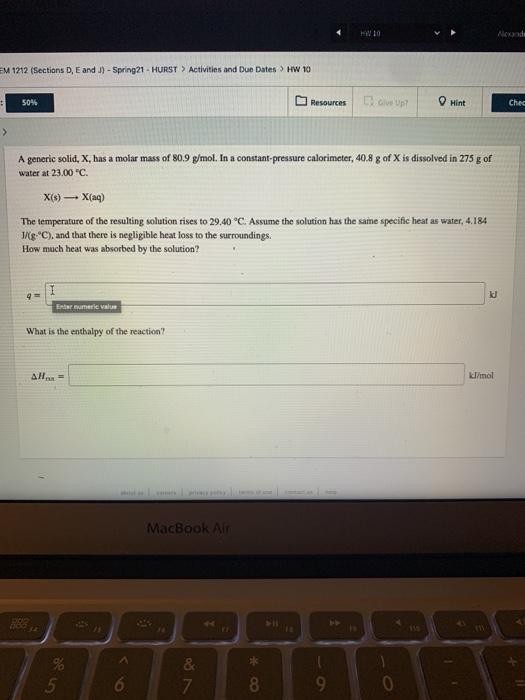
HO Alexanda EM 1212 (Sections D, E and J) - Spring 21 - HURST > Activities and Due Dates > HW 10 50% Resources I are up Hint Chec A generic solid, x, has a molar mass of 80.9 g/mol. In a constant-pressure calorimeter, 40.8 g of X is dissolved in 275 g of water at 23.00 C. X(6) X(aq) The temperature of the resulting solution rises to 29.40 C. Assume the solution has the same specific heat as water, 4.184 J/g C), and that there is negligible heat loss to the surroundings. How much heat was absorbed by the solution? k Endermere value What is the enthalpy of the reaction? AH kl/mol MacBook Air & 7 * 00 5 6. 8 9 0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


