Question: A graduate engineer did some laboratory filtration experiments to predict performance of a filter C a C O 3 slurry filter. Table 2 shows results
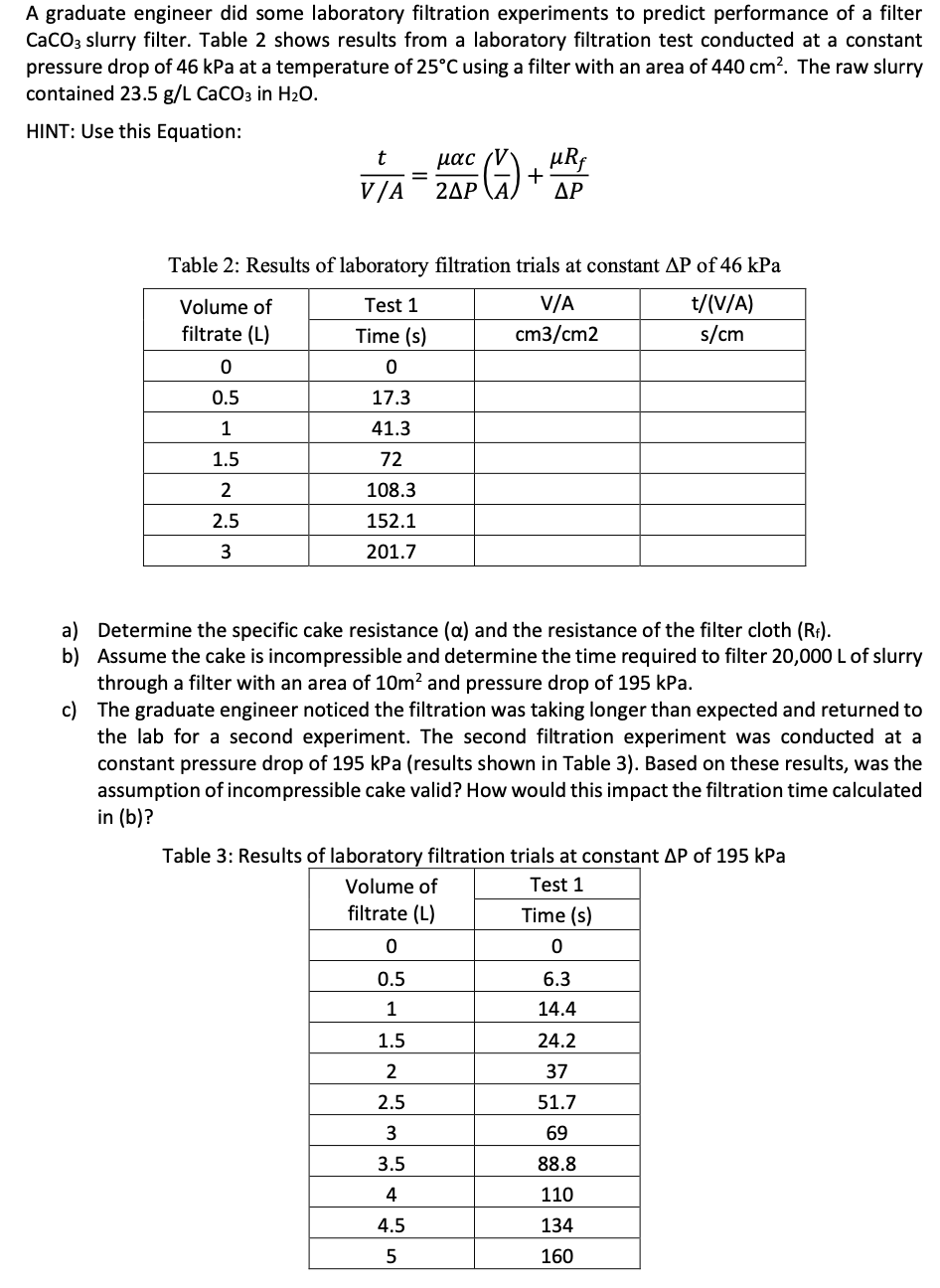
A graduate engineer did some laboratory filtration experiments to predict performance of a filter
slurry filter. Table shows results from a laboratory filtration test conducted at a constant
pressure drop of kPa at a temperature of using a filter with an area of The raw slurry
contained in
HINT: Use this Equation:
Table : Results of laboratory filtration trials at constant of kPa
a Determine the specific cake resistance and the resistance of the filter cloth
b Assume the cake is incompressible and determine the time required to filter of slurry
through a filter with an area of and pressure drop of kPa.
c The graduate engineer noticed the filtration was taking longer than expected and returned to
the lab for a second experiment. The second filtration experiment was conducted at a
constant pressure drop of kPa results shown in Table Based on these results, was the
assumption of incompressible cake valid? How would this impact the filtration time calculated
in b
Table : Results of laboratory filtration trials at constant of kPa.
I am mainly confused how to find viscosity so i can use the formula kpalphamucA Delta P
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


