Question: A group of physics students have been given a sample of unidentified charged particles. They are not given any hints about the particles, except
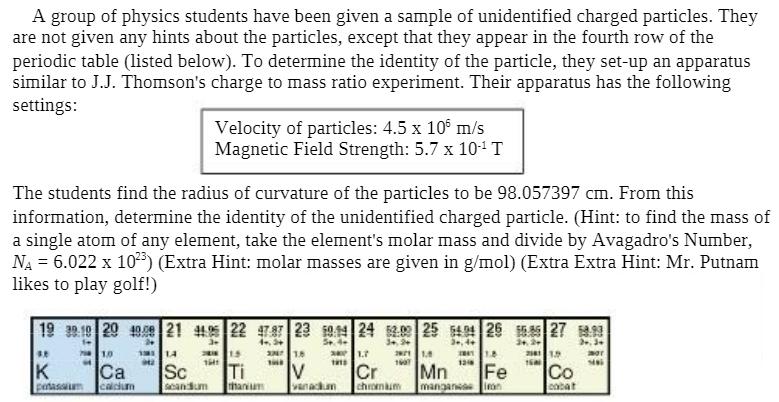
A group of physics students have been given a sample of unidentified charged particles. They are not given any hints about the particles, except that they appear in the fourth row of the periodic table (listed below). To determine the identity of the particle, they set-up an apparatus similar to J.J. Thomson's charge to mass ratio experiment. Their apparatus has the following settings: The students find the radius of curvature of the particles to be 98.057397 cm. From this information, determine the identity of the unidentified charged particle. (Hint: to find the mass of a single atom of any element, take the element's molar mass and divide by Avagadro's Number, NA = 6.022 x 103) (Extra Hint: molar masses are given in g/mol) (Extra Extra Hint: Mr. Putnam likes to play golf!) 19 32 19 20 49.09 21 4425 22 47.87 23 1944 24 52.09 25 5404 26 15 27 3 4, 3+ 5+ 3+ 3+ 4+ 3+2+ 2,3 3.8 K potassium 71.0 Velocity of particles: 4.5 x 10 m/s Magnetic Field Strength: 5.7 x 10- T Ca calcium 11 1.4 942 2810 1.5 1641 Sc sacandum thanium 3 1.8 THE :> 301.7 1915 V vanackum 2011. 1607 Cr chromium 1041 1.8 121 Mn Fe manganese iron 211 1.9 1600 Co cabat mor siai
Step by Step Solution
3.47 Rating (154 Votes )
There are 3 Steps involved in it
Calculate the chargetomass ratio qm Use the formula for the radius of curvature r in a magnetic fiel... View full answer

Get step-by-step solutions from verified subject matter experts


