Question: A heat engine using a monatomic gas follows the cycle shown in the ( p V ) diagram. The gas starts out at
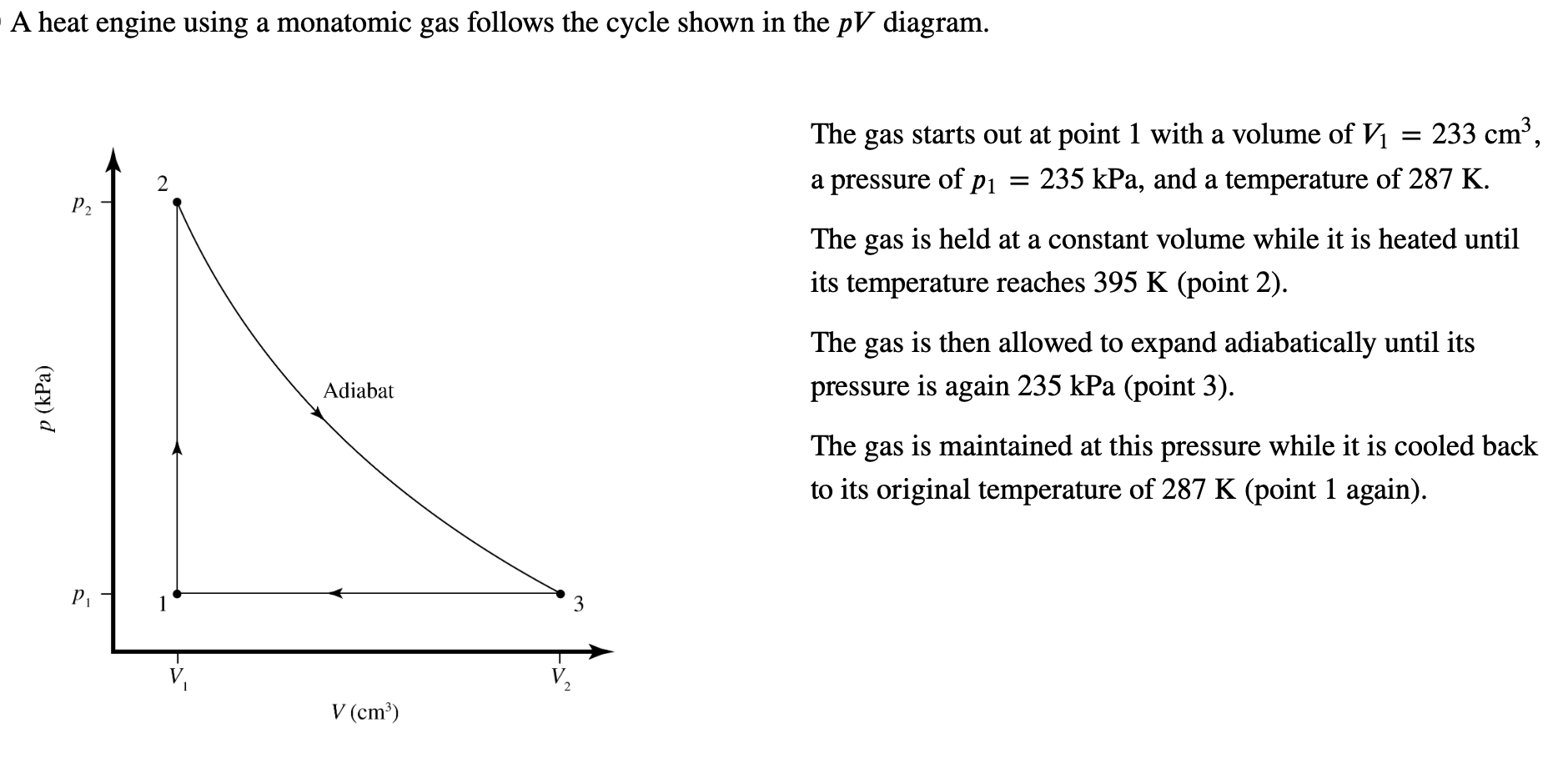
A heat engine using a monatomic gas follows the cycle shown in the p V diagram.
The gas starts out at point with a volume of Vmathrm~cm a pressure of pmathrmkPa and a temperature of K
The gas is held at a constant volume while it is heated until its temperature reaches K point
The gas is then allowed to expand adiabatically until its pressure is again kPa point
The gas is maintained at this pressure while it is cooled back to its original temperature of K point again
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


