Question: A hydrogen atom occupies a specific state with orbital angular momentum quantum number [. . : 1 L , The spin quantum numberis s =
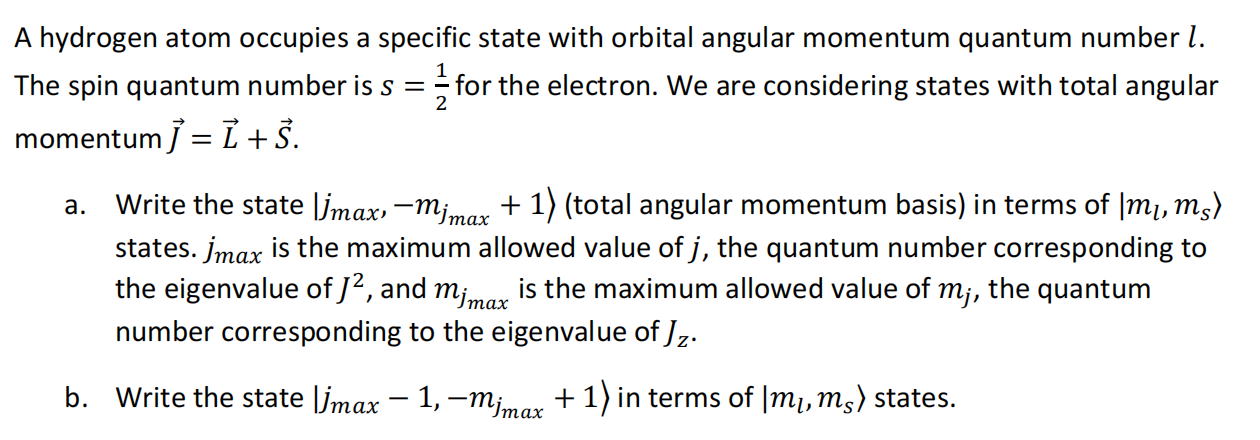
A hydrogen atom occupies a specific state with orbital angular momentum quantum number [. . : 1 L , The spin quantum numberis s = Efor the electron. We are considering states with total angular momentumf: L+5. a. Write the state |jqx, m;,_ + 1) (total angular momentum basis) in terms of |m;, my) states. jqax is the maximum allowed value of j, the quantum number corresponding to the eigenvalue of /2, and m;_ is the maximum allowed value of m;, the quantum number corresponding to the eigenvalue of /,. b. Write the state |[jmqy 1,m; _+ 1) in terms of |m;, my) states
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


