Question: A hypothetical solution forms between a solid and a liquid. The values of the thermodynamic quantities involved in the process are shown in the following
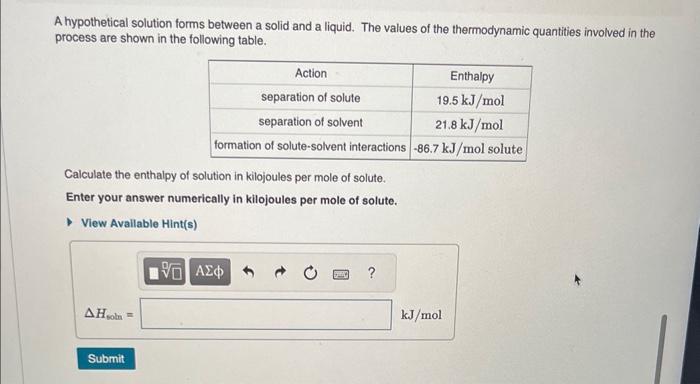
A hypothetical solution forms between a solid and a liquid. The values of the thermodynamic quantities involved in the process are shown in the following table. Calculate the enthalpy of solution in kilojoules per mole of solute. Enter your answer numerically in kilojoules per mole of solute
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


