Question: A hypothetical solution forms between a solid and a liquid. The values of the thermodynamic quantities involved in the process are shown in the following
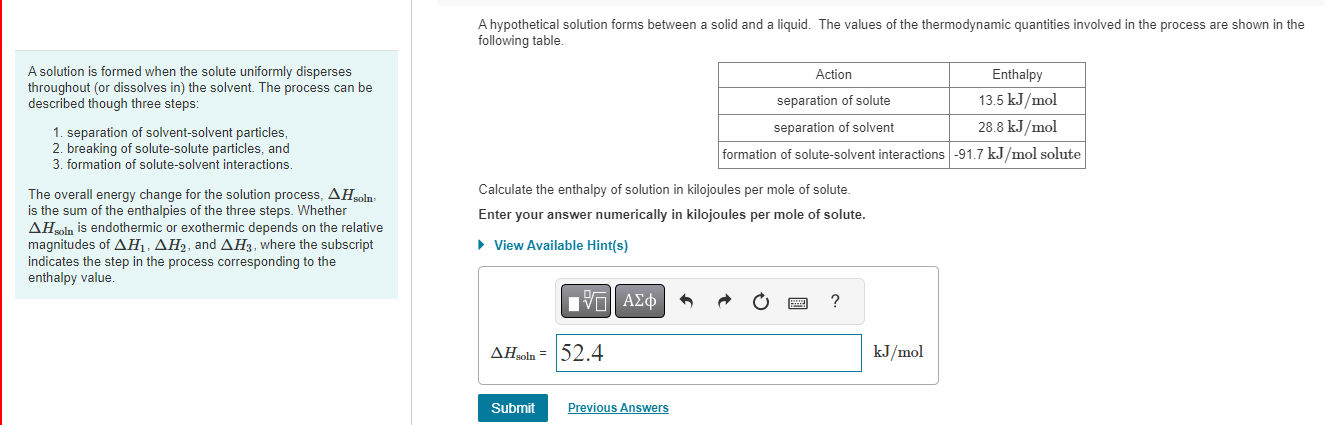
A hypothetical solution forms between a solid and a liquid. The values of the thermodynamic quantities involved in the process are shown in the following table. A solution is formed when the solute uniformly disperses throughout (or dissolves in) the solvent. The process can be described though three steps: 1. separation of solvent-solvent particles, 2. breaking of solute-solute particles, and 3. formation of solute-solvent interactions. The overall energy change for the solution process, Hsoln, Calculate the enthalpy of solution in kilojoules per mole of solute. is the sum of the enthalpies of the three steps. Whether Hsoln is endothermic or exothermic depends on the relative magnitudes of H1,H2, and H3, where the subscript indicates the step in the process corresponding to the enthalpy value
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


