Question: a) (i) Derive the Nernst equation, and explain all the terms. (3: (ii) What is the potential of a cell made up of Zn/Zn2+
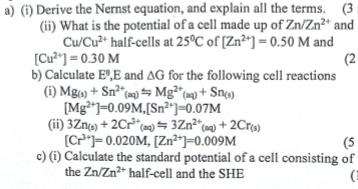

a) (i) Derive the Nernst equation, and explain all the terms. (3: (ii) What is the potential of a cell made up of Zn/Zn2+ and Cu/Cu2+ half-cells at 25C of [Zn2+]=0.50 M and [Cu2]=0.30 M b) Calculate E,E and AG for the following cell reactions (i) Mg) + Sn2(a) Mg (aq) + Sn(s) [Mg]=0.09M,[Sn2]=0.07M (ii) 3Zn(s) + 2Cr (a)=3Zn2+(aq) + 2Cr(s) [C]=0.020M, [Zn2+]=0.009M (2 (5 c) (i) Calculate the standard potential of a cell consisting of the Zn/Zn2+ half-cell and the SHE (ii) What will the e.m.f of the cell be if Zn2+-0.45M P(H)-1.0atm and [H*]=1.8M (3 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


