Question: A light source emits yellow visible light with frequency 529 THz (1 THz= 1012 Hz). Calculate the wavelength of the light in nm and
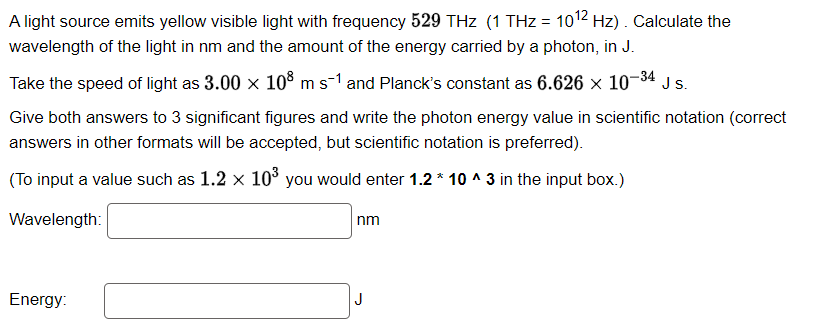
A light source emits yellow visible light with frequency 529 THz (1 THz= 1012 Hz). Calculate the wavelength of the light in nm and the amount of the energy carried by a photon, in J. Take the speed of light as 3.00 108 m s1 and Planck's constant as 6.626 10-34 Js. Give both answers to 3 significant figures and write the photon energy value in scientific notation (correct answers in other formats will be accepted, but scientific notation is preferred). (To input a value such as 1.2 10 you would enter 1.2 * 10 ^ 3 in the input box.) Wavelength: Energy: nm J
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


