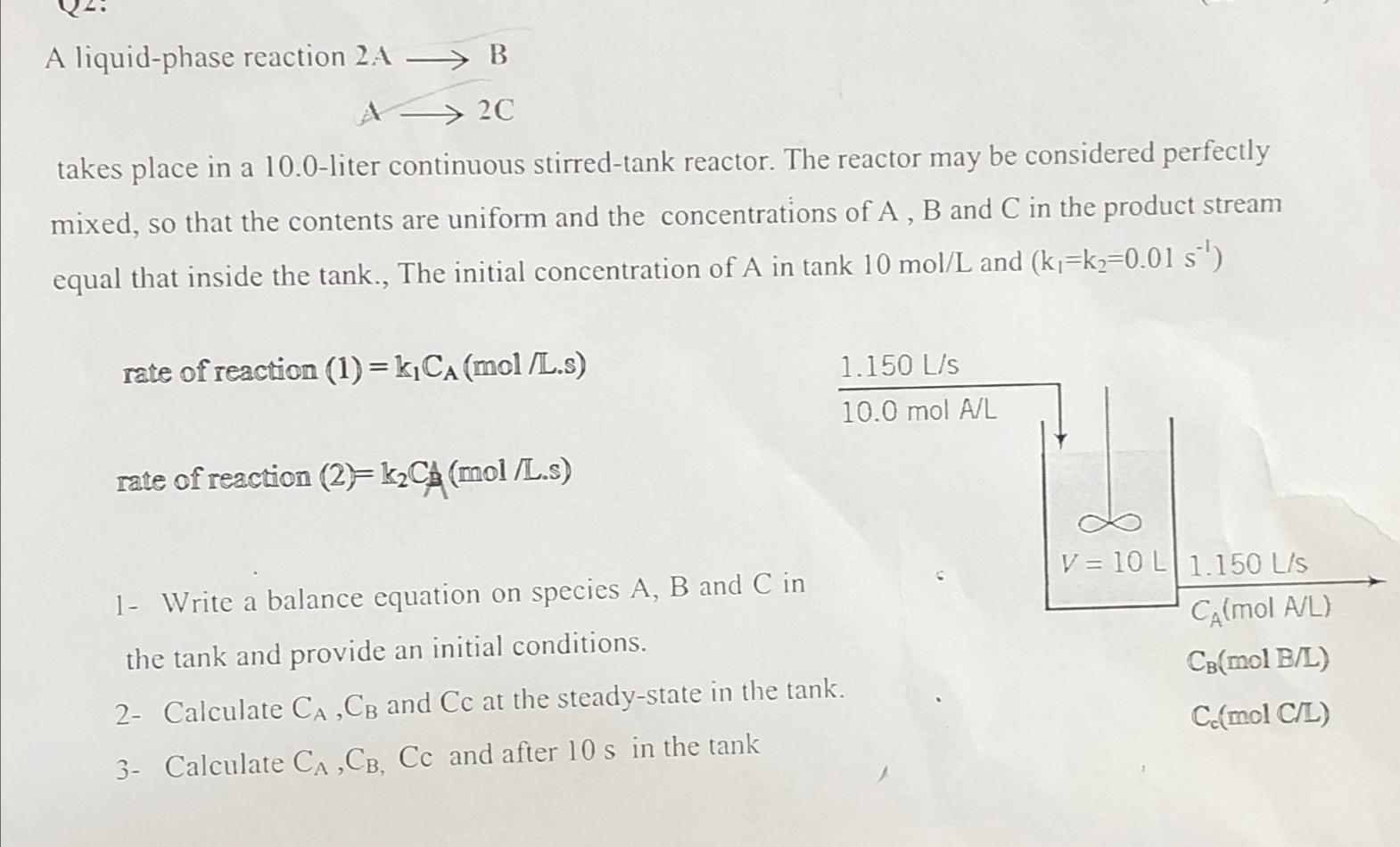
Question: A liquid - phase reaction 2 AlongrightarrowB Alongrightarrow 2 C takes place in a 1 0 . 0 - liter continuous stirred - tank reactor.
A liquidphase reaction AlongrightarrowB
Alongrightarrow
takes place in a liter continuous stirredtank reactor. The reactor may be considered perfectly mixed, so that the contents are uniform and the concentrations of A B and C in the product stream equal that inside the tank., The initial concentration of in tank and
rate of reaction
rate of reaction
Write a balance equation on species and in the tank and provide an initial conditions.
Calculate and at the steadystate in the ta
Calculate and after in the tank
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


