Question: A liquid stream containing reactants A and B undergoes the following parallel reactions: R A+ B S T where rR = 4CACB0.5 (mol L
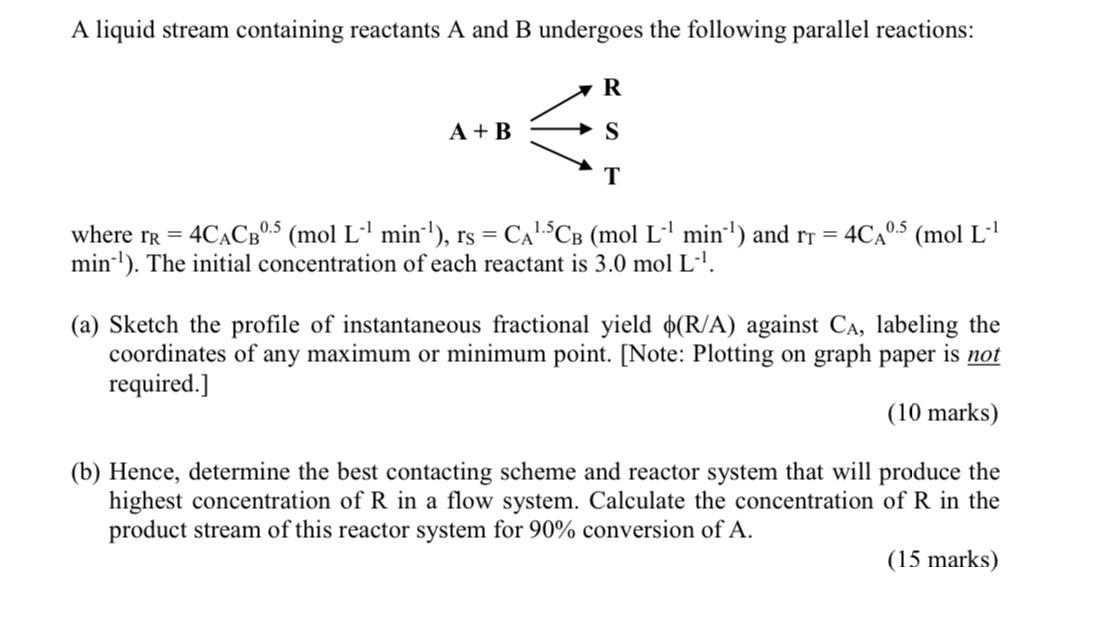
A liquid stream containing reactants A and B undergoes the following parallel reactions: R A+ B S T where rR = 4CACB0.5 (mol L min), rs = CA15CB (mol L- min) and rr = 4CA min). The initial concentration of each reactant is 3.0 mol L-. 0.5 (mol L-1 (a) Sketch the profile of instantaneous fractional yield (R/A) against CA, labeling the coordinates of any maximum or minimum point. [Note: Plotting on graph paper is not required.] (10 marks) (b) Hence, determine the best contacting scheme and reactor system that will produce the highest concentration of R in a flow system. Calculate the concentration of R in the product stream of this reactor system for 90% conversion of A. (15 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


