Question: A (made-up) metal has a face-centered cubic crystal structure. Its density is 20.5g/cm3. its molar mass is 189g/mol. What is the radius of an atom
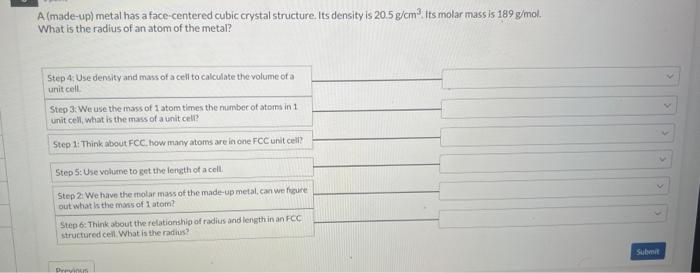
A (made-up) metal has a face-centered cubic crystal structure. Its density is 20.5g/cm3. its molar mass is 189g/mol. What is the radius of an atom of the metal
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


