Question: A metallic solid with atoms in a face-centered cubic unit ceil with an edge length of 392 pm has a density of 21.45g/cm2. a. Calculate
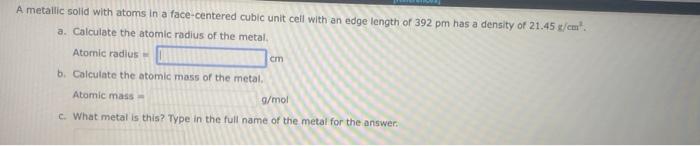

A metallic solid with atoms in a face-centered cubic unit ceil with an edge length of 392 pm has a density of 21.45g/cm2. a. Calculate the atomic radius of the metal. Atomic radius = b. Calculate the atomic mass of the metal. Atomic mass = g/mol c. What metal is this? Type in the full name of the metal for the answer. CH3CH2OCH2CH3 ) was one of the first chemicals used as an anesthetic. At 34.67C, diethyl ether has a vapor prersure of 760 , torr, and at 10.8C,ithatavaper. pressure of 301 torr, What is the AI of vaparization for diethys ether? H of vaporization = kj/imol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


