Question: A negatively charged membrane (i.e., cation exchange membrane) is able to retain anions by the Donnan exclusion mechanism. The amount of the fixed charges in
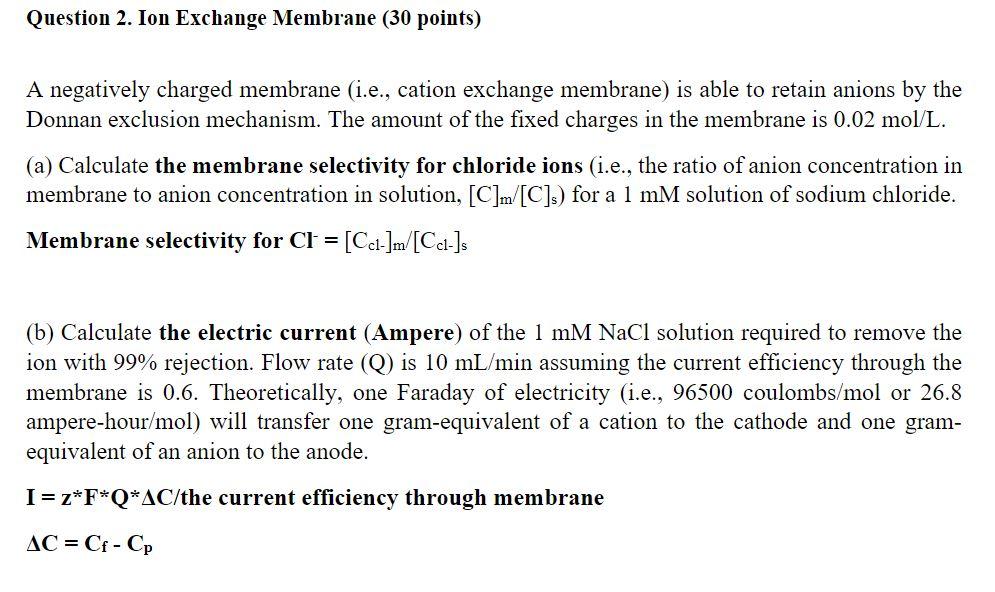
A negatively charged membrane (i.e., cation exchange membrane) is able to retain anions by the Donnan exclusion mechanism. The amount of the fixed charges in the membrane is 0.02mol/L. (a) Calculate the membrane selectivity for chloride ions (i.e., the ratio of anion concentration in membrane to anion concentration in solution, [C]m/[C]s ) for a 1mM solution of sodium chloride. Membrane selectivity for Cl=[Ccl]m/[Ccl]s (b) Calculate the electric current (Ampere) of the 1mMNaCl solution required to remove the ion with 99% rejection. Flow rate (Q) is 10mL/min assuming the current efficiency through the membrane is 0.6. Theoretically, one Faraday of electricity (i.e., 96500 coulombs /mol or 26.8 ampere-hour /mol ) will transfer one gram-equivalent of a cation to the cathode and one gramequivalent of an anion to the anode. I=zFQC/ the current efficiency through membrane C=CfCp
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


