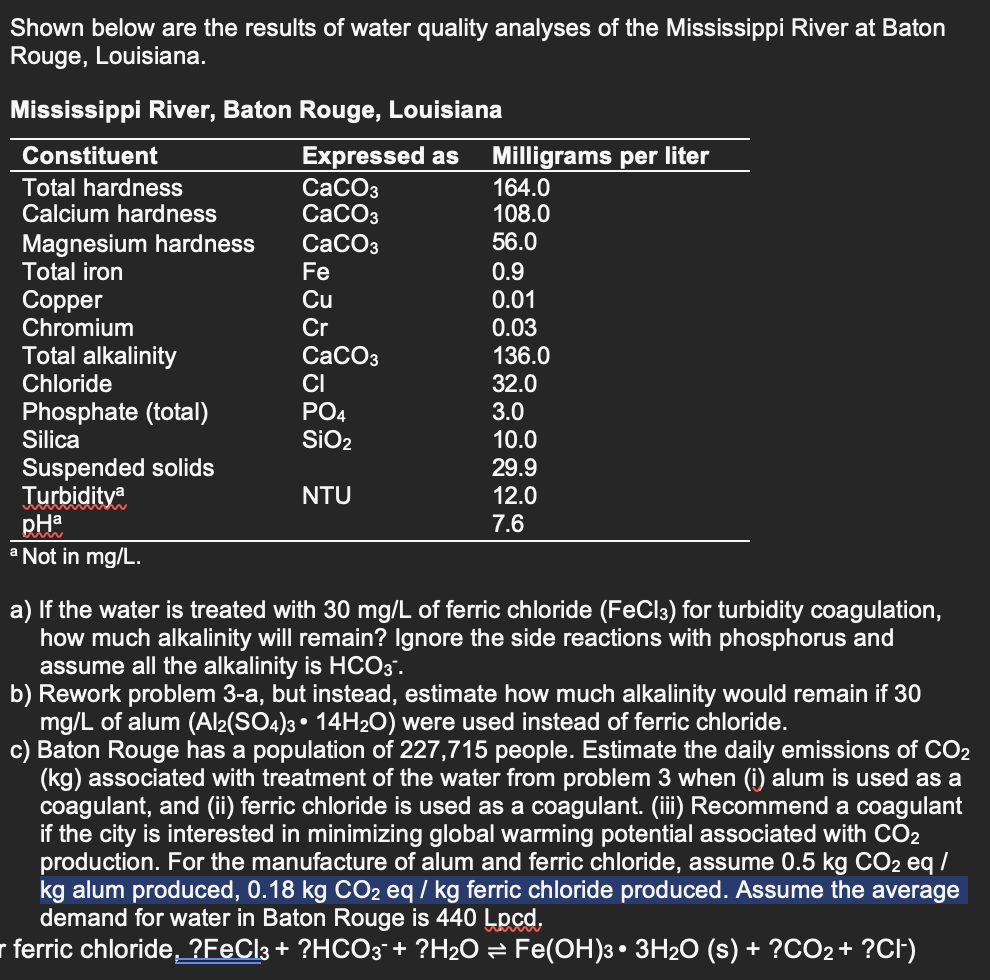
Question: ? a Not in m g L . a ) If the water is treated with 3 0 m g L of ferric chloride (
Not in
a If the water is treated with of ferric chloride for turbidity coagulation,
how much alkalinity will remain? Ignore the side reactions with phosphorus and
assume all the alkalinity is
b Rework problem a but instead, estimate how much alkalinity would remain if
of alum were used instead of ferric chloride.
c Baton Rouge has a population of people. Estimate the daily emissions of
associated with treatment of the water from problem when i alum is used as a
coagulant, and ii ferric chloride is used as a coagulant. iii Recommend a coagulant
if the city is interested in minimizing global warming potential associated with
production. For the manufacture of alum and ferric chloride, assume
kg alum produced, ferric chloride produced. Assume the average
demand for water in Baton Rouge is Lpod.
ferric chloride, :
I can't figure out c Please Help
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


