Question: A. Polar compounds can interact with water by forming. B. Examples of CH bonds and CC bonds. are peptide bonds, C. In the atoms are

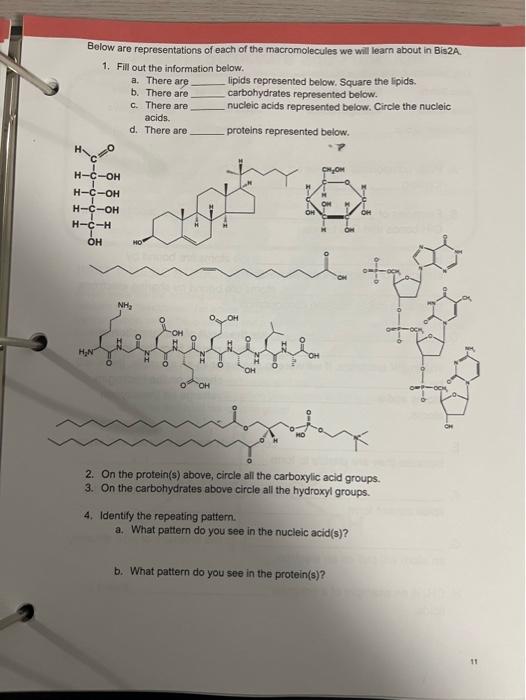
A. Polar compounds can interact with water by forming. B. Examples of CH bonds and CC bonds. are peptide bonds, C. In the atoms are bound by attraction of opposite ions, where as in , atoms are bound by sharing electrons to attain a stable electron configuration. D. are the result of the formation of a which results from the unequal sharing of electrons. If we were to use water as an example, there will be a charge on the hydrogen atoms and a charge on the oxygen atoms. E. compounds cannot interact with water because they are unable to form F. cannot interact with lipids because the lipids cannot form G. is the measure of how strongly an atom will pull on an electron. H. CH3 is an example of a nonpolar found on lipids. Below are representations of each of the macromolecules we will learn about in Bis2A. 1. Fill out the information below. a. There are lipids represented below. Square the lipids. b. There are carbohydrates represented below. c. There are nucleic acids represented below. Circle the nucleic acids. d. There are proteins represented below. 2. On the protein(s) above, circle all the carboxylic acid groups. 3. On the carbohydrates above circle all the hydroxyl groups. 4. Identify the repeating pattern. a. What pattern do you see in the nucleic acid(s)? b. What pattern do you see in the protein(s)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


