Question: 214 A radioactive isotope X decays by emitting two alpha (a) particles and one beta (3) to form 83Bi (a) What is the atomic
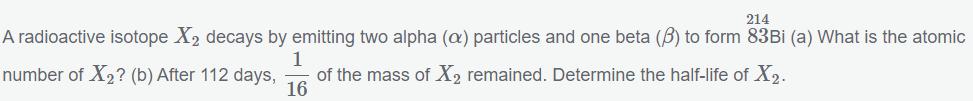
214 A radioactive isotope X decays by emitting two alpha (a) particles and one beta (3) to form 83Bi (a) What is the atomic 1 number of X? (b) After 112 days, of the mass of X remained. Determine the half-life of X. 16
Step by Step Solution
★★★★★
3.49 Rating (166 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

a The atomic number of X can be determined by examining the change in atomic number that o... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


