Question: A reaction takes place in two CSTR reactors as shown in the diagram (parallel). A -> B -r(A) = kC(A) if k = 0.314 min^(-1),
A reaction takes place in two CSTR reactors as shown in the diagram (parallel). 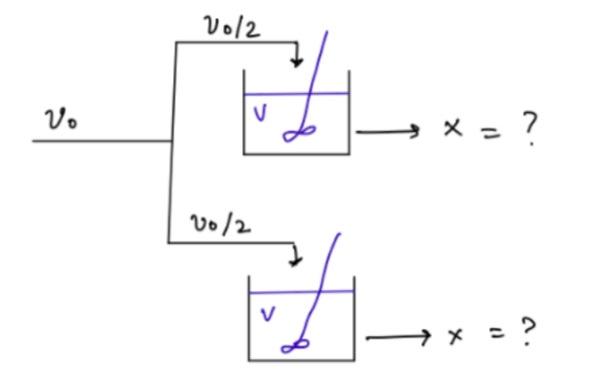
A -> B -r(A) = kC(A)
if k = 0.314 min^(-1), V = 2000 (dm^3) for both CSTR reactors & v(0) = (dm^3/min)
a) Calculate the conversion (X(parallel))
b) Determine which case would yield a higher conversion rate, X(series) = 0.85 or X(parallel)

V012 V. X = ? 10/2 Ph V + x =
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


