Question: A reactor maintained at 350 C and 2 bar is fed an equimolar mixture of ethylene and benzene in order to synthesize ethylbenzene. All the
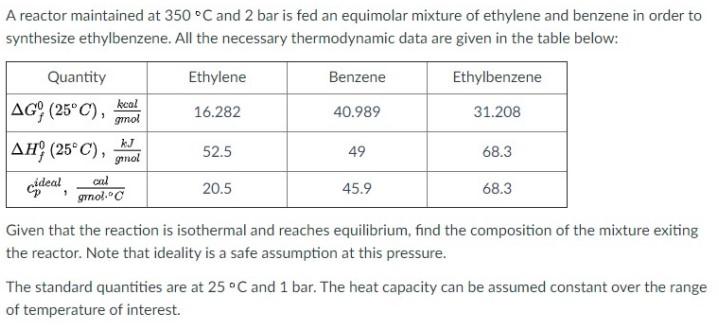
A reactor maintained at 350 C and 2 bar is fed an equimolar mixture of ethylene and benzene in order to synthesize ethylbenzene. All the necessary thermodynamic data are given in the table below: Quantity Ethylene Benzene Ethylbenzene AG (25C), 16.282 40.989 31.208 AHY (25C), gmol 52.5 49 68.3 cdeal 20.5 grol." 45.9 68.3 kcal gnol kJ 7 cal Given that the reaction is isothermal and reaches equilibrium, find the composition of the mixture exiting the reactor. Note that ideality is a safe assumption at this pressure. The standard quantities are at 25C and 1 bar. The heat capacity can be assumed constant over the range of temperature of interest
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


