Question: A reactor ( Tank 1 ) is set up with a fixed, small amount of catalyst which catalyzes the reaction A 2 B . The
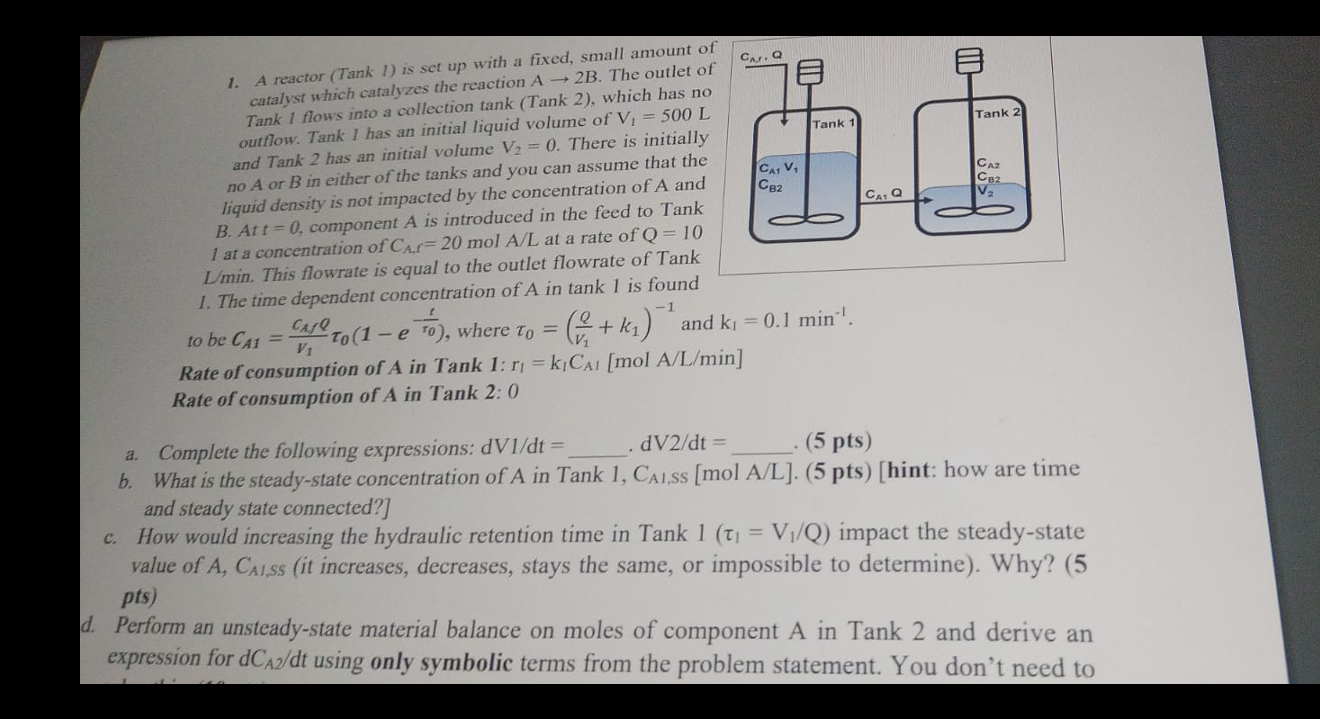
A reactor Tank is set up with a fixed, small amount of
catalyst which catalyzes the reaction The outlet of
Tank flows into a collection tank Tank which has no
outflow. Tank I has an initial liquid volume of
and Tank has an initial volume There is initially
no or in either of the tanks and you can assume that the
liquid density is not impacted by the concentration of A and
B At component is introduced in the feed to Tank
at a concentration of mol at a rate of
Lmin. This flowrate is equal to the outlet flowrate of Tank
The time dependent concentration of in tank is found
to be where and
Rate of consumption of in Tank :
Rate of consumption of in Tank :
a Complete the following expressions:
b What is the steadystate concentration of A in Tank C AISS mol AL ptshint: how are time
and steady state connected?
c How would increasing the hydraulic retention time in Tank impact the steadystate
value of it increases, decreases, stays the same, or impossible to determine Why?
pts
d Perform an unsteadystate material balance on moles of component A in Tank and derive an
expression for dt using only symbolic terms from the problem statement. You don't need to
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


