Question: A representation of the bonding in solid silver is shown below. This representation best shows which of the following? ( A ) Each silver atom
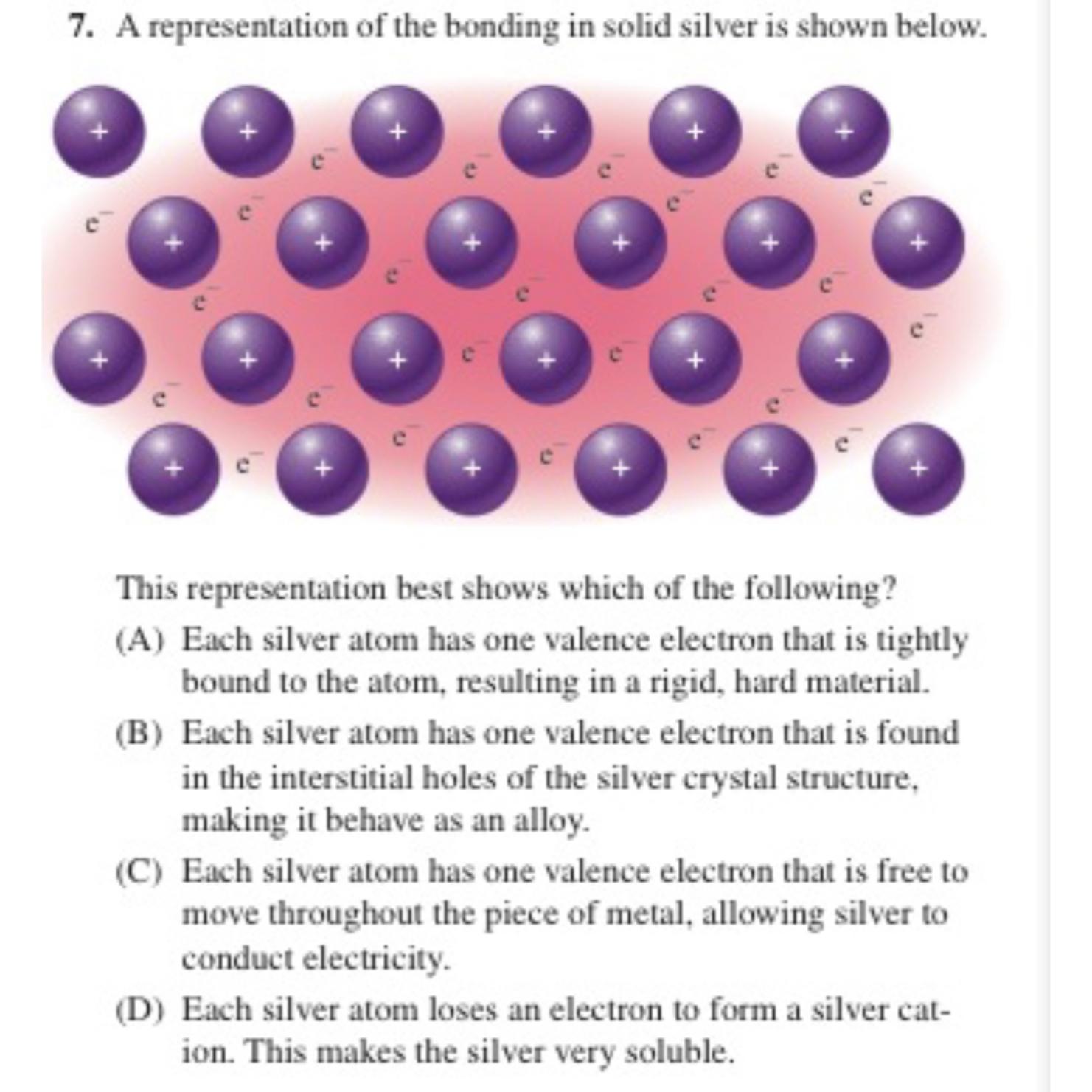
A representation of the bonding in solid silver is shown below.
This representation best shows which of the following?
A Each silver atom has one valence electron that is tightly bound to the atom, resulting in a rigid, hard material.
B Each silver atom has one valence electron that is found in the interstitial holes of the silver crystal structure, making it behave as an alloy.
C Each silver atom has one valence electron that is free to move throughout the piece of metal, allowing silver to conduct electricity.
D Each silver atom loses an electron to form a silver cation. This makes the silver very soluble.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


