Question: A reversible, first-order, exothermic reaction ( AR ) takes place in a CSTR. The CSTR, shown in Figure 1, has no heating or cooling, and
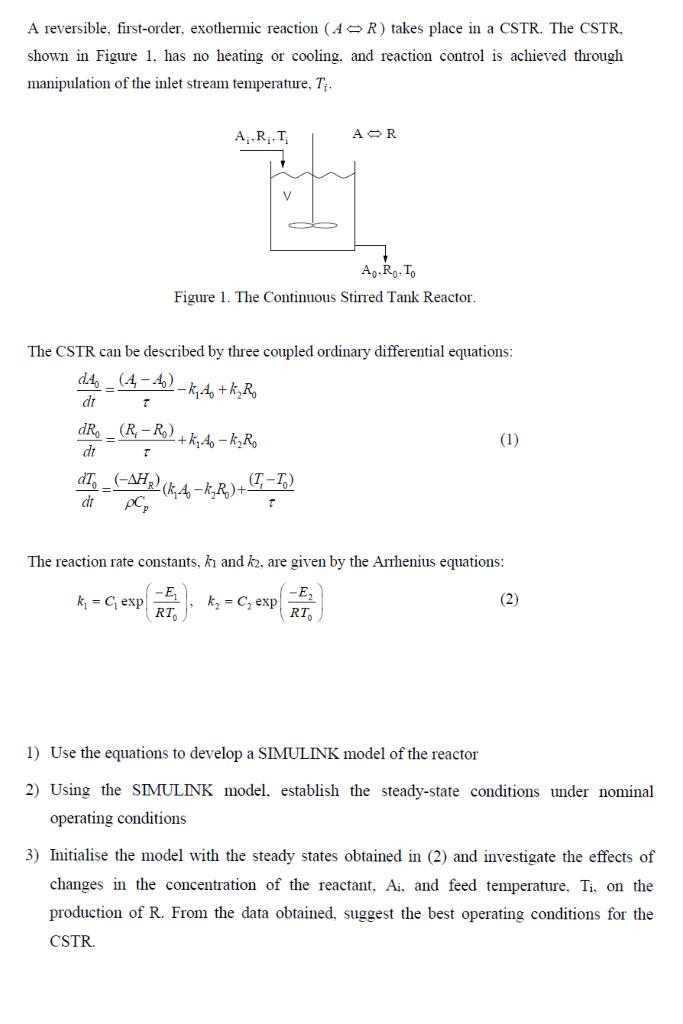

A reversible, first-order, exothermic reaction ( AR ) takes place in a CSTR. The CSTR, shown in Figure 1, has no heating or cooling, and reaction control is achieved through manipulation of the inlet stream temperature, Ti. Figure 1. The Continuous Stirred Tank Reactor. The CSTR can be described by three coupled ordinary differential equations: dtdA0dtdR0dtdT0=(A1A0)k1A0+k2R0=(RiR0)+k1A0k2R0=Cp(HR)(k1A0k2R0)+(TiT0) The reaction rate constants, k1 and k2, are given by the Arrhenius equations: k1=C1exp(RT0E1),k2=C2exp(RT0E2) 1) Use the equations to develop a SIMULINK model of the reactor 2) Using the SIMULINK model, establish the steady-state conditions under nominal operating conditions 3) Initialise the model with the steady states obtained in (2) and investigate the effects of changes in the concentration of the reactant, Ai, and feed temperature, Ti, on the production of R. From the data obtained, suggest the best operating conditions for the CSTR. A reversible, first-order, exothermic reaction (AR) takes place in a CSTR. The CSTR, shown in Figure 1, has no heating or cooling, and reaction control is achieved through mampulation of the inlet stream temperature, Tt. Figure 1. The Continuous Stirred Tank Reactor. The CSTR can be described by three compled ordinary differential equations: dtdA0dtdR0dT0=t(A1A0)k1A0+k2R0=r(R1R0)+k1A0k2R0=p(HR)(k1A0k2R0)+(T2T0) The reaction rate coustians, k and k, are given by the Anhenius equations: k1C1exp(RT2E1),k2C2exp(RT0E2) 1) Use the egnations to develop a SnMUT INK model of the reactor 2) Using the SIMULINK model, establish the steady-state conditions under noninal operating conditions 3) Initialise the model with the steady states obtained in (2) and investigate the effects of changes in the concentration of the reactant. Ai, and feed temperature, Ti. on the production of R. From the data obtained, suggest the hest operating conditions for the CSTR. Example Values of the Parameter's 0.5As1.5mol/lRq=0.0mol/l=60s1HR=5000cal/mol=1kg/lCP=1000cal/kgKR=1.987cal/molK Arrhenius Expressions for Reaction Rate Constants k1=C1exp(RT0E1)k2=C2exp(RT0E2)E1=10103E2=15103C1=5103C2=1106
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


