Question: A sample containing 2.0 moles of He is expanded from 22.8 to 31.7 dm3 at 22deg C (isothermally) against zero external pressure. Calculate Q,
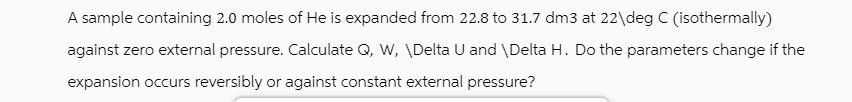
A sample containing 2.0 moles of He is expanded from 22.8 to 31.7 dm3 at 22\deg C (isothermally) against zero external pressure. Calculate Q, W, \Delta U and \Delta H. Do the parameters change if the expansion occurs reversibly or against constant external pressure?
Step by Step Solution
★★★★★
3.36 Rating (159 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

For an isothermal expansion of an ideal gas we can use the following equations Work done W in an iso... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


