Question: A second order liquid-phase reaction (AB+C) is occurred in an isothermal fixed-bed reactor at 303K. Initial concentration of A is 2mol/m3. An experiment was done
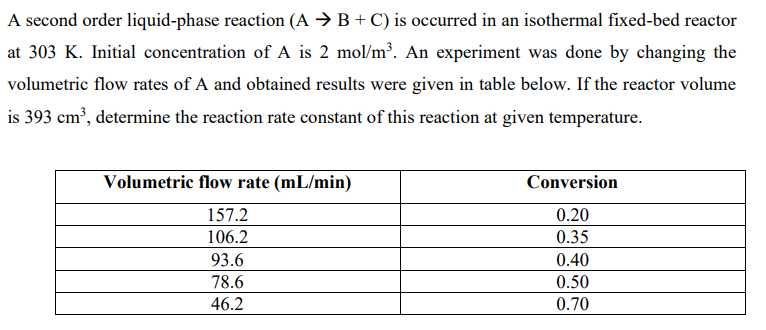
A second order liquid-phase reaction (AB+C) is occurred in an isothermal fixed-bed reactor at 303K. Initial concentration of A is 2mol/m3. An experiment was done by changing the volumetric flow rates of A and obtained results were given in table below. If the reactor volume is 393cm3, determine the reaction rate constant of this reaction at given temperature
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


