Question: A simple way to estimate whether a molecule is a strong electrolyte, a weak electrolyte, or a nonelectrolyte is to examine an equation that accurately
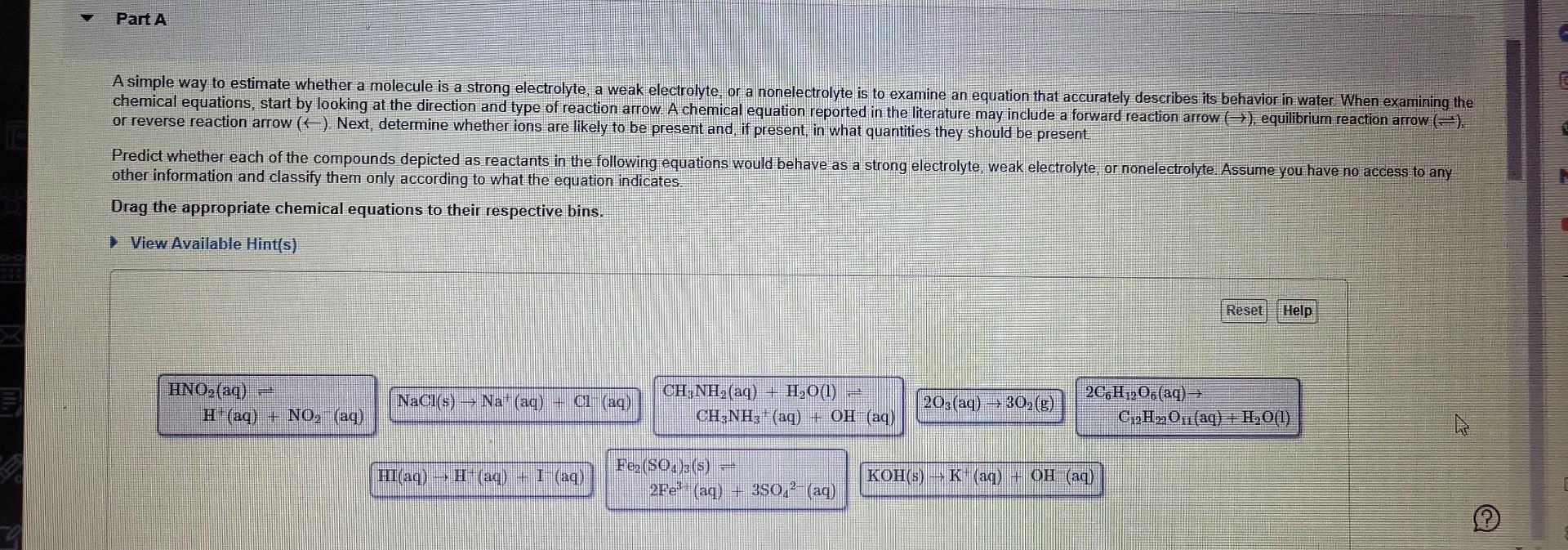
A simple way to estimate whether a molecule is a strong electrolyte, a weak electrolyte, or a nonelectrolyte is to examine an equation that accurately describes its behavior in water When examining the or reverse reaction arrow (). Next, determine whether ions are likely to be present and, if present, in what quantifies they should be present Predict whether each of the compounds depicted as reactants in the following equations would behave as a strong electrolyte, weak electrolyte, or nonelectrolyte. Assume you have no access to any other information and classify them only according to what the equation indicates. Drag the appropriate chemical equations to their respective bins
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


