Question: A Solid Oxide Fuel Cell (SOFC) system is proposed as shown below. The system utilizes methane (CH4) and air. All stream conditions are given in
A Solid Oxide Fuel Cell (SOFC) system is proposed as shown below. The system utilizes methane (CH4) and air. All stream conditions are given in the diagram below. Across the SOFC, assume that only hydrogen is consumed in the electrochemical reactions with 100% hydrogen utilization; carbon monoxide passes through the SOFC without change. Within the SOFC, O2- ions move through the electrolyte from the cathode to the anode. The flow rate of air at the cathode inlet is determined such that the ratio of the flow rate of the fuel stream (1) to the air stream is stoichiometric.
a) Assume that the stream (1) at the exit of the reformer/water gas shift reactor consists of CO, CO2, H2 and H2O. The products are at thermodynamic equilibrium at the given temperature and the pressure. At equilibrium, there are 0.049 kmol/s of CO2 and 0.284 kmol/s of H2O within the product stream (1). How much methane and water do we need to inject into the reformer expressed in [kmol/s]? (Note that the partial pressure based equilibrium constant of the water-gas shift reaction at 800 C is Kp=0.8879) b) What are the flow rates of hydrogen [kmol/s] and the current produced by the SOFC [A]? c) How much heat transfer Q1 do we need across the reformer/water gas shift reactor [kJ/s]? d) What is the flow rate of air into the SOFC [kmol/s]?
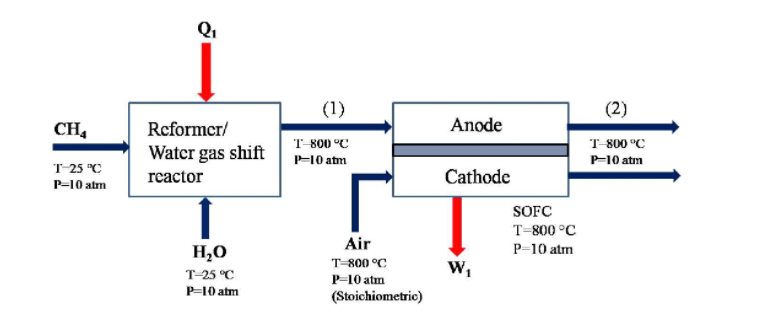
Q. (1) (2) CH4 Anode Rcformer/ Water gas shift reactor T 800 C P-10 atm T 800 C P-10 atm T 250 P-10 atm 1 Cathode SOFC T=800C P-10 atm W HO T-25 C P=10 atm Air T-800C P-10 atn (Stoichiometric) Q. (1) (2) CH4 Anode Rcformer/ Water gas shift reactor T 800 C P-10 atm T 800 C P-10 atm T 250 P-10 atm 1 Cathode SOFC T=800C P-10 atm W HO T-25 C P=10 atm Air T-800C P-10 atn (Stoichiometric)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


